Abstract
The genome of pancreatic ductal adenocarcinoma (PDAC) frequently contains deletions of tumour suppressor gene loci, most notably SMAD4, which is homozygously deleted in nearly one-third of cases1. As loss of neighbouring housekeeping genes can confer collateral lethality, we sought to determine whether loss of the metabolic gene malic enzyme 2 (ME2) in the SMAD4 locus would create cancer-specific metabolic vulnerability upon targeting of its paralogous isoform ME3. The mitochondrial malic enzymes (ME2 and ME3) are oxidative decarboxylases that catalyse the conversion of malate to pyruvate and are essential for NADPH regeneration and reactive oxygen species homeostasis2,3. Here we show that ME3 depletion selectively kills ME2-null PDAC cells in a manner consistent with an essential function for ME3 in ME2-null cancer cells. Mechanistically, integrated metabolomic and molecular investigation of cells deficient in mitochondrial malic enzymes revealed diminished NADPH production and consequent high levels of reactive oxygen species. These changes activate AMP activated protein kinase (AMPK), which in turn directly suppresses sterol regulatory element-binding protein 1 (SREBP1)-directed transcription of its direct targets including the BCAT2 branched-chain amino acid transaminase 2) gene. BCAT2 catalyses the transfer of the amino group from branched-chain amino acids to α-ketoglutarate (α-KG)4 thereby regenerating glutamate, which functions in part to support de novo nucleotide synthesis. Thus, mitochondrial malic enzyme deficiency, which results in impaired NADPH production, provides a prime ‘collateral lethality’ therapeutic strategy for the treatment of a substantial fraction of patients diagnosed with this intractable disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Bardeesy, N. et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 20, 3130–3146 (2006)
Pongratz, R. L., Kibbey, R. G., Shulman, G. I. & Cline, G. W. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J. Biol. Chem. 282, 200–207 (2007)
Jiang, P., Du, W., Mancuso, A., Wellen, K. E. & Yang, X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 493, 689–693 (2013)
Hutson, S. M., Fenstermacher, D. & Mahar, C. Role of mitochondrial transamination in branched chain amino acid metabolism. J. Biol. Chem. 263, 3618–3625 (1988)
Muller, F. L., Aquilanti, E. A. & DePinho, R. A. Collateral lethality: a new therapeutic strategy in oncology. Trends Cancer 1, 161–173 (2015)
Muller, F. L. et al. Passenger deletions generate therapeutic vulnerabilities in cancer. Nature 488, 337–342 (2012)
Witkiewicz, A. K. et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 6, 6744 (2015)
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014)
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012)
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013)
Ying, H. et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 30, 355–385 (2016)
Holecek, M. Relation between glutamine, branched-chain amino acids, and protein metabolism. Nutrition 18, 130–133 (2002)
Suryawan, A. et al. A molecular model of human branched-chain amino acid metabolism. Am. J. Clin. Nutr. 68, 72–81 (1998)
Hutson, S. M., Sweatt, A. J. & Lanoue, K. F. Branched-chain amino acid metabolism: implications for establishing safe intakes. J. Nutr. 135 (Suppl.), 1557S–1564S (2005)
O’Connell, T. M. The complex role of branched chain amino acids in diabetes and cancer. Metabolites 3, 931–945 (2013)
Mayers, J. R. et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 20, 1193–1198 (2014)
Fearon, K. C., Glass, D. J. & Guttridge, D. C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 16, 153–166 (2012)
Tsoli, M. & Robertson, G. Cancer cachexia: malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol. Metab. 24, 174–183 (2013)
Sweatt, A. J. et al. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am. J. Physiol. Endocrinol. Metab. 286, E64–E76 (2004)
Krebs, H. A. & Lund, P. Aspects of the regulation of the metabolism of branched-chain amino acids. Adv. Enzyme Regul. 15, 375–394 (1976)
Hutson, S. M., Cree, T. C. & Harper, A. E. Regulation of leucine and alpha-ketoisocaproate metabolism in skeletal muscle. J. Biol. Chem. 253, 8126–8133 (1978)
Blättler, S. M., Rencurel, F., Kaufmann, M. R. & Meyer, U. A. In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase. Proc. Natl Acad. Sci. USA 104, 1045–1050 (2007)
Woods, A. et al. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J. Biol. Chem. 278, 28434–28442 (2003)
Li, Y. et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13, 376–388 (2011)
Porstmann, T. et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 (2008)
Tontonoz, P., Kim, J. B., Graves, R. A. & Spiegelman, B. M. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol. 13, 4753–4759 (1993)
Berg, J. M., Tymoczko, J. L. & Stryer, L. Biochemistry 7th edn ( W.H. Freeman, 2012)
Doench, J. G. et al. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat. Biotechnol. 32, 1262–1267 (2014)
Heckl, D. et al. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat. Biotechnol. 32, 941–946 (2014)
Dey, P., Ström, A. & Gustafsson, J. A. Estrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene 33, 4213–4225 (2014)
Kapoor, A. et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell 158, 185–197 (2014)
Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011)
Paik, J. H. et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5, 540–553 (2009)
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012)
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protocols 7, 562–578 (2012)
Orsomando, G. et al. Simultaneous single-sample determination of NMNAT isozyme activities in mouse tissues. PLoS One 7, e53271 (2012)
Acknowledgements
We thank T. Tieu for vector cloning; the MD Anderson core facilities, including K. Dunner Jr for High Resolution Electron Microscopy Facility, Sequencing and Microarray Facility (SMF), Flow Cytometry and Cellular Imaging Core Facility; S. Jiang and Z. Xu for assistance in maintenance of mouse colonies; Z. Lu for discussion; and D. Spring for editing. This study was supported by NCI P01 CA117969 grant (R.A.D.); UT Star award (R.A.D.); CPRIT grant RP140612 (R.A.D.); DOD Postdoctoral research fellowship W81XWH-14-1-0429 (P.D.); MD Anderson Bridge Fund (R.A.D.); St. Louis Ovarian Cancer Awareness Research Grant (D.N.) and Odyssey Fellowships at MD Anderson (D.Z., T.G.). The MD Anderson core facilities are supported by NIH P30 CA16672.
Author information
Authors and Affiliations
Contributions
P.D., Y.A.W., D.N. and R.A.D. designed the studies, interpreted the data and wrote the manuscript; P.D. performed all experiments; J.B. performed experiments and analysis of metabolite isotope tracing, Seahorse and UPLC; A.A. and L.Y. conducted metabolomics data analysis; C.C.W. performed bioinformatics analysis; W.-T.L. and H.W. conducted tissue microarray analysis; Z.L. conducted ChIP analysis; T.G. was responsible for ME2 CRISPR design and cloning; Y.K., J.F. and A.V. contributed essential reagents and resources; F.M., G.G., H.Y., G.D. and A.M. provided intellectual input; A.C., N.S., D.Z., Y.K., J.L. and E.C. provided technical support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks C. Van Dang, A. Trumpp and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 ME2 is codeleted with SMAD4 in pancreatic cancer.
a, b, Scatterplots of SMAD4 mRNA expression against log2 CNA of all tumour types from CCLE datasets (a; n = 877), and PDAC samples in TCGA (b; n = 149). c, d, Scatterplots of ME2 mRNA expression against log2 CNA of all tumour types from CCLE datasets (c; n = 877) and PDAC samples from CCLE datasets (d; n = 46). e, log2 CNA of ME2 in TCGA PDAC database analysed by Oncomine (n = 131). f, Correlation between mRNA expression of ME2 and SMAD4 in TCGA PDAC database (n = 149). g, CNA of ME2 and SMAD4 in UTSW microdissected PDAC samples7 (n = 109) as reported by cBioportal were current in August 2016. h, Representative IHC images of SMAD4 and ME2 expression in PDAC samples compared with a matching normal pancreas sample. i, j, Additional ME2 (i) and SMAD4 (j) IHC images of PDAC samples. Staining is shown as no stain (score 0) and low-to-high staining (score 1). k, IHC analysis of paired normal and PDAC samples (n = 62) for ME2 and SMAD4 expression. Scoring is based on no expression (score 0) and low-to-high expression (score 1).
Extended Data Figure 2 ME1 and ME3 are paralogous isoforms of ME2.
a, Representative IHC images of ME3 in PDAC samples. Staining is shown as no stain or low-to-high staining. b, IGV image of chromosome 11 encompassing region q14 of PDAC cell lines from CCLE (n = 46). c, ME3 mRNA expression against log2 copy number of PDAC lines from CCLE (n = 46). d, Expression of ME1 upon depletion of ME1 in PATU8988T cells. β-Actin used as loading control. e, Colony-formation assay of cell lines corresponding to the immunoblot in d. f, Quantification of the colony-formation assay in e. g, Representative microscopic fields of PATU8988T shCtrl and shME1#3 cells (scale bar, 100 μm). h, Expression of ME1 upon depletion of ME1 in KP-1NL cells. i, Colony-formation assay corresponding to the immunoblot in h. j, Quantification of the colony-formation assay in i. k, Representative microscopic fields of KP-1NL shCtrl and shME1#3 cells (scale bar, 100 μm). l, Colony-formation assay of NHDF-Neo cells (skin fibroblast cell line) (ishCtrl ± dox, ishME1#3 ± dox and NHDF-Neo/ishME3#1 ± dox). β-Actin used as loading control. Error bars represent s.d. of at least n = 3 replicates.
Extended Data Figure 3 ME3 depletion in ME2-null cells leads to growth inhibition.
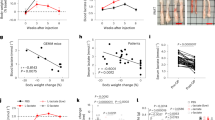
a, Immunoblot showing expression of ME3 upon depletion with three independent Dox-inducible hairpins or non-targeting inducible control hairpin (ishCtrl) in PATU8988T cells. ME1 expression remained unchanged upon ME3 depletion. b, Expression of ME3 following depletion by ishME3#1 and ishCtrl hairpin in BxPC3 cells (ME2-null). c, Immunoblot of KP-1NL cells (ME2-intact) assessing deletion of ME3 expression. d, Immunoblot of Panc1 cells (ME2-intact) assessing deletion of ME3 expression. e, Raw photos of colony-formation assay upon depletion of ME3 in ME2-null PATU8988T cells. f, Malic enzyme activity assay upon depletion of ME3 in PATU8988T cells. g, Representative microscopic field comparing cell growth between ishCtrl ± Dox and ishME3#1 ± Dox cells (scale bar, 50 μm). h, Growth curve upon ME3 depletion. i, Raw photos of colony-formation assay upon depletion of ME3 in ME2-null BxPC3 cells. j, Raw photo of colony-formation assay upon inducible CRISPR/Cas9 deletion of ME3 in Hs766T cells (ME2-null). k, Inducible CRISPR/Cas9 deletion of ME3 inhibits colony formation in Hs766T cells. l, m, Raw photos of colony-formation assay upon depletion of ME3 in ME2-intact (l) KP-1NL and (m) Panc1 cells. n, o, Raw tumour image after 60 days of tumour growth (n) and graph of mean tumour weights (o) (n = 5). p, Immunoblot of ME3-depleted (dox+) and non-depleted (dox−) xenograft tumour samples confirms complete depletion of ME3. Human-specific ME1 antibody did not detect any remaining ME1 protein in dox+ mouse tumours #3 and #4, indicating no remaining human tumour cells. Red asterisk denotes the specific band. β-Actin used as loading control. q, Luciferase imaging (IVIS spectrum) of nude mice 79 days after orthotopic transplantation of PATU8988T-ishME3 cells (±dox). Colour scale, minimum 274, maximum 2,986. r, Survival data for mice (n = 10 each group) orthotopically grafted with PATU8988T cells (ishCtrl ± dox and ishME3#1 ± dox).
Extended Data Figure 4 Inhibition of ME3 in ME2-null cells affects tumour growth.
a, b, Representative IHC and haematoxylin and eosin-stained images of xenograft tumour samples of PATU8988T ishCtrl ± dox (a) and ishME3 ± dox (b) cells. Arrowheads indicate Ki67-positive cells. c, Top pathways enriched in ME3-depleted xenograft tumours from Ingenuity pathway analysis of RNA-seq data. d, Electron transport cycle (ETC) pathways are enriched in non-ME3-depleted versus ME3-depleted xenograft tumours as analysed by GSEA.
Extended Data Figure 5 ME3 depletion in ME2-null cells increases apoptosis.
a, Increase in annexin V staining upon ME3 depletion. b, Raw flow cytometric plot of rate of apoptosis using annexin V and propidium iodide staining of PATU8988T cells. c, qRT–PCR data showing ME2 (CMV-GFP-ME2) expression in PATU8998T-ishME3#1 cells. d, Immunoblot of overexpression of ME2 in ME3-depleted PATU8988T cells. e, Malic enzyme assay showing the rescue of enzyme activity upon overexpression of ME2 in PATU8998T-ishME3#1 cells. f, Colony-formation assay of cell lines (PATU8998T-ishME3#1-GFP and PATU8998T-ishME3#1-GFP-ME2). g, Representative xenograft tumour photo showing partial rescue of tumour growth upon ME2 overexpression in ME3-depleted cell lines (n = 5, each group). h, GSEA analysis of RNA-seq data showing enrichment of PGC1α signature. i, GSEA analysis of RNA-seq data showing enrichment of TCA cycle signature. j, Colony-formation data for PATU8988T-ishME3 cells rescued with GSH (4 mM) or NAC (4 mM). k, Expression of pAMPK1-T172, BCAT2 and ME3 upon treatment with Glc (10 mM), Gln (2 mM) and/or Pyr (5 mM). l, Raw flow cytometric plot of DCFDA upon rescue with pyruvate (5 mM). m, Relative DCFDA fluorescence upon rescue with pyruvate. n, Colony-formation data for PATU8988T-ishME3 cells rescued with pyruvate (5 mM). o, Mapping of carbon atom transitions using uniformly labelled 13C5-glutamine. p, Mass isotopomer distribution (MID) of uniformly labelled 13C5-glutamine contribution to TCA cycle metabolites. ME3 depletion led to increased glutamine flux into TCA cycle. Error bars represent s.e.m. of n = 4 biological samples from two independent experiments. P values were determined by two-tailed t-test.
Extended Data Figure 6 ME3 depletion in ME2-null cells causes mitochondrial dysfunction.
a, Mapping of carbon atom transitions using uniformly labelled 13C6-glucose. b, MID of uniformly labelled 13C6-glucose contribution to TCA cycle metabolites. ME3 depletion led to decreased glucose entry to TCA cycle. Error bars represent the s.e.m. of n = 4 biological samples from two independent experiments. P values were determined by two-tailed t-test. c–f, Glucose uptake rate and lactate secretion rate were measured in PATU8988T (c, d) and BxPC3 (e, f) cells. g, h, Measurements of oxygen consumption rate (OCR) (g) and extracellular acidification rate (ECAR) (h) in PATU8988T cells upon ME3 depletion. i, j, Measurements of OCR (i) and ECAR (j) in KP-1NL cells upon ME3 depletion. Error bars represent s.e.m. of at least n = 5 replicates. P values were determined by two-tailed t-test. k, MitoTracker red, DAPI and F-actin staining of ME3-depleted (Dox+) and non-depleted (Dox−) cells. l–o, Glucose uptake rate and lactate secretion rate were measured in KP-1NL (l, m) and Panc1 (n, o) cells. p, Quantification of MitoTracker green staining to assess the mitochondrial biomass (scale bar, 10 μm). q, Representative flow cytometry data of MitoTracker green staining of ME3-depleted (+Dox) versus non-depleted cells. Error bars represent s.e.m. of at least n = 5 replicates. P values were determined by two-tailed t-test.
Extended Data Figure 7 Malic enzymes affect branched-chain amino acid metabolism.
a, b, Glutamine uptake rate measured in PATU8988T (a) and KP-1NL (b) cells upon ME3 depletion. c, d, Measurement of amino acid uptake and secretion rates in PATU8988T (c) and KP-1NL (d) cells upon ME3 depletion. Positive values refer to amino acid uptake; negative values refer to secretion. Error bars represent s.e.m. of n = 6 biological samples (PATU8988T and KP-1NL). P values were determined by two-tailed t-test.
Extended Data Figure 8 Malic enzymes regulate BCAT2 via a ROS-mediated pathway.
a, Schematic of the first enzymatic step of BCAA catabolism to branched-chain ketoacid (BCKA). b, Time course of expression of BCAT2, ME3, pAMPKα-T172 and total AMPK following ME3 depletion in PATU8988T cells. c, Expression of BCAT2 and ME3 in cells treated with another independent ishRNA (ishME3#3). d, Expression of BCAT2 and ME3 in BxPC3 cells. e, Expression of BCAT2 and ME3 upon depletion of ME1 and ME3 using independent (non-dox dependent) shRNAs. f, Expression of ME3 and BCAT2 upon siRNA depletion of ME3 in PDAC lines. β-Actin used as loading control. g, h, Raw flow cytometry data of DCFDA-stained cells for measurement of ROS in PATU8988T (g) and BxPC3 (h) cells upon ME3 depletion. i, Raw flow cytometry data of MitoSOX staining in PATU8988T and ME3-depleted PATU8988T cells. Antimycin A used as positive control. j, Immunoblot showing time course of expression of NRF2 in cells with dox-induced ME3 depletion. β-Actin used as loading control. k, IHC images showing NRF2 staining in ME3-depleted and control xenograft tumours (scale bar, 50 μm).
Extended Data Figure 9 Increase in ROS activates AMPK pathway.
a, b, Measurement of NADPH (a) and total ROS (b) in ME2-rescued and ME3-depleted PATU8988T cells. Error bars represent s.d. of at least n = 5 replicates. c, Immunoblot of ME3, BCAT2 and AMPK expression upon depletion of ME3 followed by Trolox treatment for 24 h. Structure of Trolox (above), a synthetic vitamin E analogue that acts as a potent antioxidant. d, Expression of BCAT2 in PATU8988T and Panc1 cells upon treatment with AICAR for 14 h. e, Colony-formation assay showing decreased cell growth upon shRNA-mediated depletion of BCAT2 and SREBP1 using two independent shRNAs. Error bars represent s.d. of at least n = 3 replicates. f, OCR in cells depleted of BCAT2 by shRNA. g, OCR in cells upon overexpression of BCAT2. Error bars represent s.e.m. of at least n = 5 replicates. P values were determined by two-tailed t-test.
Extended Data Figure 10 BCAAs contribution to nucleotide synthesis.
a–c, MID of 15N-labelled BCAA (Leu) contribution to glutamate (a), alanine (b), and serine (c). d, Plot of 13C-labelled BCAAs contribution to TCA cycle metabolites. Error bars represent s.e.m. of n = 4 biological samples from two independent experiments. P values were determined by two-tailed t-test. e, Colony-formation assay of cells treated with nucleotides (mix of thymine, guanine, cytosine, uracil, and inosine, 250 μM each) showing rescue of ME3-depleted PATU8988T cells. Error bars represent s.d. of n = 3 biological samples from two independent experiments. P values were determined by two-tailed t-test.
Supplementary information
Supplementary Figures
This file contains the raw data for the Figures and Extended Data Figures (see Contents for details) and additional references. (PDF 3690 kb)
Supplementary Table
This table contains the tumor progression data. (XLSX 60 kb)
Rights and permissions
About this article
Cite this article
Dey, P., Baddour, J., Muller, F. et al. Genomic deletion of malic enzyme 2 confers collateral lethality in pancreatic cancer. Nature 542, 119–123 (2017). https://doi.org/10.1038/nature21052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21052
This article is cited by
-
AKT1 phosphorylation of cytoplasmic ME2 induces a metabolic switch to glycolysis for tumorigenesis
Nature Communications (2024)
-
SIRT5-mediated ME2 desuccinylation promotes cancer growth by enhancing mitochondrial respiration
Cell Death & Differentiation (2024)
-
Suppression of the human malic enzyme 2 modifies energy metabolism and inhibits cellular respiration
Communications Biology (2023)
-
The role of ROS in tumour development and progression
Nature Reviews Cancer (2022)
-
Reduced nicotinamide adenine dinucleotide phosphate in redox balance and diseases: a friend or foe?
Acta Pharmacologica Sinica (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.