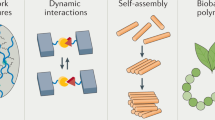
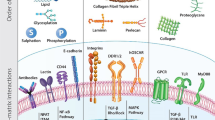
Abstract
The vast opportunities for biomaterials design and functionality enabled by mimicking nature continue to stretch the limits of imagination. As both biological understanding and engineering capabilities develop, more sophisticated biomedical materials can be synthesized that have multifaceted chemical, biological and physical characteristics designed to achieve specific therapeutic goals. Mimicry is being used in the design of polymers for biomedical applications that are required locally in tissues, systemically throughout the body, and at the interface with tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rosales, A. M. & Anseth, K. S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nature Rev. Mater. 1, 15012 (2016).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). This is a landmark paper on the role of a hydrogel scaffold's mechanical properties in stem-cell differentiation.
Nelson, C. M., VanDuijn, M. M., Inman, J. L., Fletcher, D. A. & Bissell, M. J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298–300 (2006).
Ye, C. et al. Self-(un)rolling biopolymer microstructures: Rings, tubules, and helical tubules from the same material. Angew. Chem. Int. Edn Engl. 54, 8490–8493 (2015).
Benoit, D. S. W., Schwartz, M. P., Durney, A. R. & Anseth, K. S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nature Mater. 7, 816–823 (2008).
Lin, C. C. & Anseth, K. S. Cell-cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing beta-cell function. Proc. Natl Acad. Sci. USA 108, 6380–6385 (2011).
Lutolf, M. P. & Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnol. 23, 47–55 (2005). Pioneering study describing how synthetic hydrogels can be used to mimic the native extracellular matrix.
Lee, T. T. et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nature Mater. 14, 352–360 (2015).
Martino, M. M. et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343, 885–888 (2014).
DeForest, C. A. & Tirrell, D. A. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nature Mater. 14, 523–531 (2015).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature Mater. 15, 326–334 (2016).
Dingal, P. C. D. P. et al. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nature Mater. 14, 951–960 (2015).
Beck, J. N., Singh, A., Rothenberg, A. R., Elisseeff, J. H. & Ewald, A. J. The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials 34, 9486–9495 (2013).
Chaudhuri, O. et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nature Mater. 13, 970–978 (2014).
Johnson, R. & Halder, G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nature Rev. Drug Discov. 13, 63–79 (2014).
Sun, Y. et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nature Mater. 13, 599–604 (2014).
Sur, S., Matson, J. B., Webber, M. J., Newcomb, C. J. & Stupp, S. I. Photodynamic control of bioactivity in a nanofiber matrix. ACS Nano 6, 10776–10785 (2012).
Kloxin, A. M., Kasko, A. M., Salinas, C. N. & Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009).
Gilbert, T. W., Sellaro, T. L. & Badylak, S. F. Decellularization of tissues and organs. Biomaterials 27, 3675–3683 (2006).
Badylak, S. F. & Gilbert, T. W. Immune response to biologic scaffold materials. Semin. Immunol. 20, 109–116 (2008).
Sadtler, K. et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352, 366–370 (2016).
Ali, O. A., Tayalia, P., Shvartsman, D., Lewin, S. & Mooney, D. J. Inflammatory cytokines presented from polymer matrices differentially generate and activate DCs. Adv. Funct. Mater. 23, 4621–4628 (2013). This is the first study to incorporate immunological cytokines into a biomaterial scaffold to modulate an immune response.
Kim, J. et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nature Biotechnol. 33, 64–72 (2015).
Ahn, B. K., Lee, D. W., Israelachvili, J. N. & Waite, J. H. Surface-initiated self-healing of polymers in aqueous media. Nature Mater. 13, 867–872 (2014).
Damo, M., Wilson, D. S., Simeoni, E. & Hubbell, J. A. TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma. Sci. Rep. 5, 17622 (2015).
Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nature Mater. 14, 643–651 (2015).
Leslie, D. C. et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nature Biotechnol. 32, 1134–1140 (2014). This paper introduces surface topographies from nature to control biomaterial surface properties.
Vegas, A. J. et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nature Biotechnol. 34, 345–352 (2016). This pioneering study demonstrates that a combinatorial library approach of constructing synthetic alginate variants can lead to biomaterials that reduce foreign-body reactions in non-human primates for at least 6 months.
Park, K.-C. et al. Condensation on slippery asymmetric bumps. Nature 531, 78–82 (2016).
Vegas, A. J. et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nature Med. 22, 306–311 (2016).
Singh, A. et al. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nature Mater. 13, 988–995 (2014).
Kelich, J. M. et al. Super-resolution imaging of nuclear import of adeno-associated virus in live cells. Mol. Ther. Meth. Clin. Dev. 2, 15047 (2015).
Goldsmith, C. S. & Miller, S. E. Modern uses of electron microscopy for detection of viruses. Clin. Microbiol. Rev. 22, 552–563 (2009).
Sachse, C. et al. High-resolution electron microscopy of helical specimens: a fresh look at tobacco mosaic virus. J. Mol. Biol. 371, 812–835 (2007).
Vestergaard, G. et al. Stygiolobus rod-shaped virus and the interplay of crenarchaeal rudiviruses with the CRISPR antiviral system. J. Bacteriol. 190, 6837–6845 (2008).
Bharat, T. A. et al. Structural dissection of Ebola virus and its assembly determinants using cryo-electron tomography. Proc. Natl Acad. Sci. USA 109, 4275–4280 (2012).
Häring, M. et al. Virology: independent virus development outside a host. Nature 436, 1101–1102 (2005).
Jiang, X. et al. Plasmid-templated shape control of condensed DNA-block copolymer nanoparticles. Adv. Mater. 25, 227–232 (2013). This paper establishes that the shape of polymeric, plasmid DNA-containing nanoparticles can be controlled by solvent polarity, and that anisotropic biomimetic particles can have enhanced gene-delivery efficacy in vivo.
Hanson, M. C., Bershteyn, A., Crespo, M. P. & Irvine, D. J. Antigen delivery by lipid-enveloped PLGA microparticle vaccines mediated by in situ vesicle shedding. Biomacromolecules 15, 2475–2481 (2014).
Roberts, R. A. et al. Towards programming immune tolerance through geometric manipulation of phosphatidylserine. Biomaterials 72, 1–10 (2015).
Perry, J. L., Herlihy, K. P., Napier, M. E. & Desimone, J. M. PRINT: a novel platform toward shape and size specific nanoparticle theranostics. Acc. Chem. Res. 44, 990–998 (2011).
Hu, C. M. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108, 10980–10985 (2011). This paper uses cell membranes to camouflage and functionalize polymeric nanoparticles, opening the door to new hybrid biomimetic particles.
Fang, R. H. et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 14, 2181–2188 (2014).
Hu, C.-M. J. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Hu, C.-M. J., Fang, R. H., Copp, J., Luk, B. T. & Zhang, L. A biomimetic nanosponge that absorbs pore-forming toxins. Nature Nanotechnol. 8, 336–340 (2013).
Parodi, A. et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nature Nanotechnol. 8, 61–68 (2013); published online 16 December 2012.
Xuan, M., Shao, J., Dai, L., He, Q. & Li, J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv. Healthcare Mater. 4, 1645–1652 (2015).
Lai, P.-Y., Huang, R.-Y., Lin, S.-Y., Lin, Y.-H. & Chang, C.-W. Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv. 5, 98222–98230 (2015).
Rodriguez, P. L. et al. Minimal 'self' peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 339, 971–975 (2013).
Tsai, R. K., Rodriguez, P. L. & Discher, D. E. Self inhibition of phagocytosis: the affinity of 'marker of self' CD47 for SIRPalpha dictates potency of inhibition but only at low expression levels. Blood Cells Mol. Dis. 45, 67–74 (2010).
Sosale, N. G. et al. Cell rigidity and shape override CD47's “self”-signaling in phagocytosis by hyperactivating myosin-II. Blood 125, 542–552 (2015).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nature Rev. Mater. 1, 16014 (2016).
Meyer, R. A. et al. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small 11, 1519–1525 (2015).
Perica, K. et al. Enrichment and expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy. ACS Nano 9, 6861–6871 (2015).
Sunshine, J. C., Perica, K., Schneck, J. P. & Green, J. J. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials 35, 269–277 (2014).
Lashof-Sullivan, M. M. et al. Intravenously administered nanoparticles increase survival following blast trauma. Proc. Natl Acad. Sci. USA 111, 10293–10298 (2014).
Anselmo, A. C. et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 8, 11243–11253 (2014).
Chan, L. W. et al. A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci. Transl. Med. 7, 277ra29 (2015).
Kumar, V. A., Wickremasinghe, N. C., Shi, S. & Hartgerink, J. D. Nanofibrous snake venom hemostat. ACS Biomater. Sci. Eng. 1, 1300–1305 (2015).
Lee, H., Dellatore, S. M., Miller, W. M. & Messersmith, P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007).
Maier, G. P., Rapp, M. V., Waite, J. H., Israelachvili, J. N. & Butler, A. Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science 349, 628–632 (2015).
Papanna, R. et al. Cryopreserved human amniotic membrane and a bioinspired underwater adhesive to seal and promote healing of iatrogenic fetal membrane defect sites. Placenta 36, 888–894 (2015).
Zhao, Q. et al. Underwater contact adhesion and microarchitecture in polyelectrolyte complexes actuated by solvent exchange. Nature Mater. 15, 407–412 (2016).
Lee, Y. et al. Bioinspired nanoparticulate medical glues for minimally invasive tissue repair. Adv. Healthcare Mater. 4, 2587–2596 (2015).
Roche, E. T. et al. A light-reflecting balloon catheter for atraumatic tissue defect repair. Sci. Transl. Med. 7, 306ra149 (2015).
Busscher, H. J. et al. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 4, 153rv10 (2012).
Geim, A. K. et al. Microfabricated adhesive mimicking gecko foot-hair. Nature Mater. 2, 461–463 (2003).
Lee, H., Lee, B. P. & Messersmith, P. B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 448, 338–341 (2007).
Mahdavi, A. et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl Acad. Sci. USA 105, 2307–2312 (2008).
Yang, S. Y. et al. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nature Commun. 4, 1702 (2013).
Chen, M., Briscoe, W. H., Armes, S. P. & Klein, J. Lubrication at physiological pressures by polyzwitterionic brushes. Science 323, 1698–1701 (2009).
Liu, G. et al. Hairy polyelectrolyte brushes-grafted thermosensitive microgels as artificial synovial fluid for simultaneous biomimetic lubrication and arthritis treatment. ACS Appl. Mater. Interfaces 6, 20452–20463 (2014).
Sicari, B. M. et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 6, 234ra58 (2014).
Beachley, V. Z. et al. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nature Methods 12, 1197–1204 (2015).
Oliva, N. et al. Regulation of dendrimer/dextran material performance by altered tissue microenvironment in inflammation and neoplasia. Sci. Transl. Med. 7, 272ra11 (2015). This paper introduces the challenge posed by diverse physiological environments and shows they affect the responses of biomaterials in people.
Banquy, X., Burdyńska, J., Lee, D. W., Matyjaszewski, K. & Israelachvili, J. Bioinspired bottle-brush polymer exhibits low friction and amontons-like behavior. J. Am. Chem. Soc. 136, 6199–6202 (2014).
Acknowledgements
The authors thank K. Sadtler for contributions and the design of Figs 1 and 4, C. Cherry for editorial assistance, and M. Frisk for critical review and manuscript contributions. J.J.G. was supported in part by the NIH (1R01EB016721). J.H.E. was supported by the Department of Defense including the Armed Forces Institute of Regenerative Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Green, J., Elisseeff, J. Mimicking biological functionality with polymers for biomedical applications. Nature 540, 386–394 (2016). https://doi.org/10.1038/nature21005
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21005
This article is cited by
-
Reinforced levan-based electrospun nanofibers for application as adhesive scaffolds for tissue engineering
Biotechnology and Bioprocess Engineering (2024)
-
Recent advances in modified poly (lactic acid) as tissue engineering materials
Journal of Biological Engineering (2023)
-
Digital micelles of encoded polymeric amphiphiles for direct sequence reading and ex vivo label-free quantification
Nature Chemistry (2023)
-
Engineering of an electrically charged hydrogel implanted into a traumatic brain injury model for stepwise neuronal tissue reconstruction
Scientific Reports (2023)
-
Site-selected in situ polymerization for living cell surface engineering
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.