Abstract
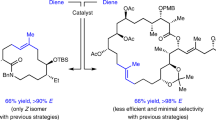
Macrocyclic compounds are central to the development of new drugs, but preparing them can be challenging because of the energy barrier that must be surmounted in order to bring together and fuse the two ends of an acyclic precursor such as an alkene (also known as an olefin)1. To this end, the catalytic process known as ring-closing metathesis (RCM)2,3,4 has allowed access to countless biologically active macrocyclic organic molecules, even for large-scale production5. Stereoselectivity is often critical in such cases: the potency of a macrocyclic compound can depend on the stereochemistry of its alkene; alternatively, one isomer of the compound can be subjected to stereoselective modification (such as dihydroxylation6). Kinetically controlled Z-selective RCM reactions have been reported7,8,9,10, but the only available metathesis approach for accessing macrocyclic E-olefins entails selective removal of the Z-component of a stereoisomeric mixture by ethenolysis10, sacrificing substantial quantities of material if E/Z ratios are near unity. Use of ethylene can also cause adventitious olefin isomerization—a particularly serious problem when the E-alkene is energetically less favoured. Here, we show that dienes containing an E-alkenyl–B(pinacolato) group, widely used in catalytic cross-coupling11, possess the requisite electronic and steric attributes to allow them to be converted stereoselectively to E-macrocyclic alkenes. The reaction is promoted by a molybdenum monoaryloxide pyrrolide complex and affords products at a yield of up to 73 per cent and an E/Z ratio greater than 98/2. We highlight the utility of the approach by preparing recifeiolide (a 12-membered-ring antibiotic)12,13 and pacritinib (an 18-membered-ring enzyme inhibitor)14,15, the Z-isomer of which is less potent than the E-isomer16. Notably, the 18-membered-ring moiety of pacritinib—a potent anti-cancer agent that is in advanced clinical trials for treating lymphoma and myelofibrosis—was prepared by RCM carried out at a substrate concentration 20 times greater than when a ruthenium carbene was used.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Martí-Centelles, V., Pandey, M. D., Burguete, M. I. & Luis, S. V. Macrocyclization reactions: the importance of conformational, configurational, and template-induced preorganization. Chem. Rev. 115, 8736–8834 (2015)
Hanson, P. R., Maitram, S., Chegondi, R. & Markley, J. L. in Handbook of Metathesis Vol. 2 (eds Grubbs, R. H. & O’Leary, D. J. ) 1–170 (Wiley–VCH, 2014)
Mallinson, J. & Collins, I. Macrocycles in new drug discovery. Future Med. Chem. 4, 1409–1438 (2012)
Gradillas, A. & Perez-Castells, J. in Metathesis in Natural Product Synthesis (eds Cossy, J., Arseniyades, S. & Meyer, C. ) 149–182 (Wiley–VCH, 2010)
Higman, C. S., Lummiss, J. A. M. & Fogg, D. E. Olefin metathesis at the dawn of implementation in pharmaceutical and specialty-chemicals manufacturing. Angew. Chem. Int. Edn 55, 3552–3565 (2016)
Li, H., Wu, J., Luo, J. & Dai, W. M. A concise total synthesis of amphidinolide T2. Chemistry 16, 11530–11534 (2010)
Hoveyda, A. H. Evolution of catalytic stereoselective olefin metathesis: from ancillary transformation to purveyor of stereochemical utility. J. Org. Chem. 79, 4763–4792 (2014)
Yu, M. et al. Synthesis of macrocyclic natural products by catalyst-controlled stereoselective ring-closing metathesis. Nature 479, 88–93 (2011)
Wang, C. et al. Efficient and selective formation of macrocyclic disubstituted Z alkenes by ring-closing metathesis (RCM) reactions catalyzed by Mo- or W-based monoaryloxide pyrrolide (MAP) complexes: applications to total syntheses of epilachnene, yuzu lactone, ambrettolide, epothilone C, and nakadomarin A. Chemistry 19, 2726–2740 (2013)
Marx, V. M., Herbert, M. B., Keitz, B. K. & Grubbs, R. H. Stereoselective access to Z and E macrocycles by ruthenium-catalyzed Z-selective ring-closing metathesis and ethenolysis. J. Am. Chem. Soc. 135, 94–97 (2013)
Carboni, B. & Carreaux, F. in Boronic Acids (ed. Hall, D. G. ) 343–376 (Wiley–VCH, 2008)
Vesonder, R. F., Stodola, F. H., Wickerham, L. J., Ellis, J. J. & Rohwedder, W. K. 11-Hydroxy-trans-8-dodenoic acid lactone, a 12-membered-ring compound from a fungus. Can. J. Chem. 49, 2029–2032 (1971)
Fürstner, A. & Langemann, K. Macrocycles by ring-closing metathesis. Synthesis 1997, 792–803 (1997)
William, A. D. et al. Discovery of macrocycle 11-(2-pyrrolidin-1-yl-ethoxy-14,19-dioxa-5,7,26-triaza-tetracyclo[19.13.1.1(2,6).1(8,12)]heptacosa-1 (25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a potent janus kinase 2/Fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor for the treatment of myelofibrosis and lymphoma. J. Med. Chem. 54, 4638–4658 (2011)
Verstovsek, S. et al. Pacritinib. Drugs Future 38, 375–386 (2013)
Poulsen, A., William, A. D. & Dymock, B. W. in RSC Drug Discovery Series No. 40 (ed. Levin, J. ) 141–205 (Wiley–VCH, 2014)
Nguyen, T. T. et al. Kinetically controlled E-selective catalytic olefin metathesis. Science 352, 569–575 (2016)
Townsend, E. M. et al. High oxidation state molybdenum imido heteroatom-substituted alkylidene complexes. Organometallics 32, 4612–4617 (2013)
Kiesewetter, E. T. et al. Synthesis of Z-(pinacolato)allylboron and Z-(pinacolato)alkenylboron compounds through selective catalytic cross metathesis. J. Am. Chem. Soc. 135, 6026–6029 (2013)
Speed, A. W. H., Mann, T. J., O’Brien, R. V., Schrock, R. R. & Hoveyda, A. H. Catalytic Z-selective cross-metathesis in complex molecule synthesis: a convergent stereoselective route to disorazole C1 . J. Am. Chem. Soc. 136, 16136–16139 (2014)
Robbins, J., Bazan, G. C., Murdzek, J. S., O’Regan, M. B. & Schrock, R. R. Reduction of molybdenum imido–alkylidene complexes in the presence of olefins to give molybdenum(IV) complexes. Organometallics 10, 2902–2907 (1991)
Schrock, R. R. & Hoveyda, A. H. Molybdenum and tungsten imido alkylidene complexes as efficient olefin-metathesis catalysts. Angew. Chem. Int. Edn 42, 4592–4633 (2003)
Koh, M. J. et al. High-value alcohols and higher-oxidation-state compounds by catalytic Z-selective cross-metathesis. Nature 517, 181–186 (2015)
Wang, Y. D., Kimball, G., Prashad, A. S. & Wang, Y. Zr-mediated hydroboration: stereoselective synthesis of vinyl boronic acid. Tetrahedr. Lett . 46, 8777–8780 (2005)
Fürstner, A. & Langemann, K. Conformationally unbiased macrocyclization reactions by using ring closing metathesis. J. Org. Chem. 61, 3942–3943 (1996)
Goldring, W. P. D., Hodder, A. S. & Weiler, L. Synthesis of macrocyclic lactams and lactones via ring-closing olefin metathesis. Tetrahedr. Lett . 39, 4955–4958 (1998)
Conrad, J. C. et al. Oligomers as intermediated in ring-closing metathesis. J. Am. Chem. Soc. 129, 1024–1025 (2007)
Jang, H., Zhugralin, A. R., Lee, Y. & Hoveyda, A. H. Highly selective methods for synthesis of internal (α-) vinylboronates through efficient NHC–Cu-catalyzed hydroboration of terminal alkynes. Utility in chemical synthesis and mechanistic basis for selectivity. J. Am. Chem. Soc. 133, 7859–7871 (2011)
Fürstner, A. & Radkowski, K. A chemo- and stereoselective reduction of cycloalkynes to (E)-cycloalkenes. Chem. Commun. (Camb.) 2182–2183 (2002)
Radkowski, K., Sundararaju, B. & Fürstner, A. A functional-group-tolerant catalytic trans hydrogenation of alkynes. Angew. Chem. Int. Edn 52, 355–360 (2013)
Brown, H. C. & Gupta, S. K. Hydroboration. XXXIX. 1,3,2-Benzodioxaborole (catecholborane) as a new hydroboration reagent for alkenes and alkynes. General synthesis of alkane- and alkeneboronic acids and esters via hydroboration. Directive effects in the hydroboration of alkenes and alkynes with catecholborane. J. Am. Chem. Soc . 97, 5249–5255 (1975)
Tucker, C. E., Davidson, J. & Knochel, P. Mild and stereoselective hydroborations of functionalized alkynes and alkenes using pinacolborane. J. Org. Chem. 57, 3482–3485 (1992)
Takai, K. et al. Transformation of aldehydes into (E)-1-alkenylboronic esters with a geminal dichromium reagent derived from a dichloromethylboronic ester and CrCl2 . Synlett 9, 963–964 (1995)
Pereira, S. & Srebnik, M. Hydroboration of alkynes with pinacolborane catalyzed by HZrCp2Cl. Organometallics 14, 3127–3128 (1995)
Morrill, C. & Grubbs, R. H. Synthesis of functionalized vinyl boronates via ruthenium-catalyzed olefin cross-metathesis and subsequent conversion to vinyl halides. J. Org. Chem. 68, 6031–6034 (2003)
Takai, K., Kunisada, Y., Tachibana, Y., Yamaji, N. & Nakatani, E. Transformation of aldehydes into (E)-1-alkenylsilanes and (E)-1-alkenylboronic esters with a catalytic amount of a chromium salt. Bull. Chem. Soc. Jpn. 77, 1581–1586 (2004)
Takaya, J., Kirai, N. & Iwasawa, N. Efficient synthesis of diborylalkenes from alkenes and diboron by a new PSiP-pincer palladium-catalyzed dehydrogenative borylation. J. Am. Chem. Soc. 133, 12980–12983 (2011)
Sun, C., Potter, B. & Morken, J. P. A catalytic enantiotopic-group-selective Suzuki reaction for the construction of chiral organoboronates. J. Am. Chem. Soc. 136, 6534–6537 (2014)
Coombs, J. R., Zhang, L. & Morken, J. P. Synthesis of vinyl boronates from aldehydes by a practical boron–Wittig reaction. Org. Lett. 17, 1708–1711 (2015)
Hong, S., Zhang, W., Liu, M., Yao, Z.-J. & Deng, W. Transition-metal-free hydroboration of terminal alkynes activated by base. Tetrahedr. Lett . 57, 1–4 (2016)
Reid, W. B., Spillane, J. J., Krause, S. B. & Watson, D. A. Direct synthesis of alkenyl boronic esters from unfunctionalized alkenes: a boryl-Heck Reaction. J. Am. Chem. Soc. 138, 5539–5542 (2016)
Ojha, D. P. & Prabhu, K. R. Pd-catalyzed hydroborylation of alkynes: a ligand controlled regioselectivity switch for the synthesis of α- or β-vinylboronates. Org. Lett. 18, 432–435 (2016)
Jee, J.-E. et al. Highly selective macrocycle formations by metathesis catalysts fixated in nanopores. J. Org. Chem. 78, 3048–3056 (2013)
van Lierop, B. J. & Fogg, D. E. On the compatibility of ruthenium metathesis catalysts with secondary phosphines. Organometallics 32, 7245–7248 (2013)
Skowerski, K., Kasprzycki, P., Bieniek, M. & Olszewski, T. K. Efficient, durable and reusable olefin metathesis catalysts with high affinity to silica gel. Tetrahedron 69, 7408–7415 (2013)
Acknowledgements
This research was supported by grants from the National Institutes of Health (GM-59426) and the National Science Foundation (CHE-1362763). M.J.K. was supported as a LaMattina Graduate Fellow in Chemical Synthesis. We thank S. Torker for many helpful discussions, and L. Ondi, J. Balazs Czirok and G. Mate Nagy for their support and advice on paraffin tablets, which were gifts from XiMo, AG.
Author information
Authors and Affiliations
Contributions
X.S., T.T.N. and M.J.K. developed the catalytic method and analysed the results regarding various catalysts and substrates. A.W.H.S. first suggested the possibility of controlling RCM stereoselectivity with an appropriate electronically deficient alkene substituent. X.S. carried out the synthesis of recifeiolide, and X.S. and D.X. performed the investigations in connection to synthesis of pacritinib. R.R.S. and A.H.H. developed the molybdenum MAP complexes used in these studies. A.H.H. directed the investigations and composed the manuscript, with revisions provided by the other authors.
Corresponding author
Ethics declarations
Competing interests
A.H.H. and R.R.S. are co-founders of a company that has licensed the technology reported here.
Extended data figures and tables
Extended Data Figure 1 By-products from RCM with an E-β-substituted styrene.
Although pathway i represents the desired route, pathways ii and ii are competitive and can lead to the formation of a variety of undesired byproducts. Mass-spectrometry analysis of the unpurified mixture resulting from the reaction of compound 1c confirmed the existence of by-products A, E, H and I and the desired RCM product. 1H-NMR analysis of the mixture confirmed the existence of stilbene (F). A, High-resolution mass spectrometry (HRMS) mass of [M+H]+, calculated for C23H35O2: 343.26370; found: 343.26277; E, HRMS[M+H]+, calculated for C44H65O4: 657.48828; found: 657.48808; H, HRMS[M+H]+, calculated for C38H61O4: 581.45730; found: 581.45698; I, HRMS[M+H]+, calculated for C29H39O2: 419.29500; found: 419.29383; RCM product, HRMS[M+H]+, calculated for C15H27O2: 239.20110; found: 239.20019.
Extended Data Figure 2 Performance of other catalyst types.
Examination of alternative Mo MAP complexes shows that the precise identity of the aryloxide ligand is crucial for achieving optimal efficiency and E selectivity. Furthermore, with two widely used achiral complexes (Mo-4 and Ru-4), efficiency and E/Z selectivity are low. Two of the more recently introduced Z-selective Ru complexes (Ru-5 and Ru-6) afford only homocoupling products. Mes, 2,4,6-(Me)3C6H2; ND, not determined; R, functional group. Reactions were performed under atmospheric nitrogen. Conversion values and E/Z ratios were determined by analysis of 1H-NMR spectra of unpurified product mixtures (± 2%). See Supplementary Information for all experimental and analytical details.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data and the NMR Spectra (see Contents for details). (PDF 4269 kb)
Rights and permissions
About this article
Cite this article
Shen, X., Nguyen, T., Koh, M. et al. Kinetically E-selective macrocyclic ring-closing metathesis. Nature 541, 380–385 (2017). https://doi.org/10.1038/nature20800
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20800
This article is cited by
-
E- and Z-trisubstituted macrocyclic alkenes for natural product synthesis and skeletal editing
Nature Chemistry (2022)
-
Air-stable 18-electron adducts of Schrock catalysts with tuned stability constants for spontaneous release of the active species
Communications Chemistry (2021)
-
Photo-mediated selective deconstructive geminal dihalogenation of trisubstituted alkenes
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.