Abstract
The Yorkie homologues YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif, also known as WWTR1), effectors of the Hippo pathway, have been identified as mediators for mechanical stimuli1. However, the role of YAP/TAZ in haemodynamics-induced mechanotransduction and pathogenesis of atherosclerosis remains unclear. Here we show that endothelial YAP/TAZ activity is regulated by different patterns of blood flow, and YAP/TAZ inhibition suppresses inflammation and retards atherogenesis. Atheroprone-disturbed flow increases whereas atheroprotective unidirectional shear stress inhibits YAP/TAZ activity. Unidirectional shear stress activates integrin and promotes integrin–Gα13 interaction, leading to RhoA inhibition and YAP phosphorylation and suppression. YAP/TAZ inhibition suppresses JNK signalling and downregulates pro-inflammatory genes expression, thereby reducing monocyte attachment and infiltration. In vivo endothelial-specific YAP overexpression exacerbates, while CRISPR/Cas9-mediated Yap knockdown in endothelium retards, plaque formation in ApoE−/− mice. We also show several existing anti-atherosclerotic agents such as statins inhibit YAP/TAZ transactivation. On the other hand, simvastatin fails to suppress constitutively active YAP/TAZ-induced pro-inflammatory gene expression in endothelial cells, indicating that YAP/TAZ inhibition could contribute to the anti-inflammatory effect of simvastatin. Furthermore, activation of integrin by oral administration of MnCl2 reduces plaque formation. Taken together, our results indicate that integrin–Gα13–RhoA–YAP pathway holds promise as a novel drug target against atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
21 December 2016
The acknowledgements sections has been updated.
References
Halder, G., Dupont, S. & Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nature Rev. Mol. Cell Biol. 13, 591–600 (2012)
Cunningham, K. S. & Gotlieb, A. I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 85, 9–23 (2005)
Yu, F. X., Zhao, B. & Guan, K. L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 (2015)
Chaqour, B. & Goppelt-Struebe, M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 273, 3639–3649 (2006)
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012)
Sorrentino, G. et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nature Cell Biol. 16, 357–366 (2014)
Zhou, J. et al. Force-specific activation of Smad1/5 regulates vascular endothelial cell cycle progression in response to disturbed flow. Proc. Natl Acad. Sci. USA 109, 7770–7775 (2012)
Tzima, E., del Pozo, M. A., Shattil, S. J., Chien, S. & Schwartz, M. A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20, 4639–4647 (2001)
Ylänne, J. et al. Mutation of the cytoplasmic domain of the integrin β3 subunit. Differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis. J. Biol. Chem. 270, 9550–9557 (1995)
Kim, C., Ye, F. & Ginsberg, M. H. Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 27, 321–345 (2011)
Vijayan, K. V. et al. Shear stress augments the enhanced adhesive phenotype of cells expressing the Pro33 isoform of integrin β3 . FEBS Lett. 540, 41–46 (2003)
Arthur, W. T., Petch, L. A. & Burridge, K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719–722 (2000)
Gong, H. et al. G protein subunit Gα13 binds to integrin αIIbβ3 and mediates integrin “outside-in” signaling. Science 327, 340–343 (2010)
Estevez, B., Shen, B. & Du, X. Targeting integrin and integrin signaling in treating thrombosis. Arterioscler. Thromb. Vasc. Biol. 35, 24–29 (2015)
Shen, B. et al. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature 503, 131–135 (2013)
Hoshiga, M., Alpers, C. E., Smith, L. L., Giachelli, C. M. & Schwartz, S. M. αvβ3 integrin expression in normal and atherosclerotic artery. Circ. Res. 77, 1129–1135 (1995)
Weng, S. et al. β3 integrin deficiency promotes atherosclerosis and pulmonary inflammation in high-fat-fed, hyperlipidemic mice. Proc. Natl Acad. Sci. USA 100, 6730–6735 (2003)
Huang, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols 4, 44–57 (2009)
Bindea, G. et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009)
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008)
Ricci, R. et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science 306, 1558–1561 (2004)
Surapisitchat, J. et al. Fluid shear stress inhibits TNF-α activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: inhibitory crosstalk among MAPK family members. Proc. Natl Acad. Sci. USA 98, 6476–6481 (2001)
Takabe, W. et al. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid. Redox Signal. 15, 1379–1388 (2011)
Zanconato, F. et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nature Cell Biol. 17, 1218–1227 (2015)
Liu, X. et al. Tead and AP1 coordinate transcription and motility. Cell Reports 14, 1169–1180 (2016)
Ma, X. et al. Impaired Hippo signaling promotes Rho1-JNK-dependent growth. Proc. Natl Acad. Sci. USA 112, 1065–1070 (2015)
Hoffmann, E., Dittrich-Breiholz, O., Holtmann, H. & Kracht, M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72, 847–855 (2002)
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015)
Wang, N. et al. Shear stress regulation of Krüppel-like factor 2 expression is flow pattern-specific. Biochem. Biophys. Res. Commun. 341, 1244–1251 (2006)
Miao, H. et al. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J. Vasc. Res. 42, 77–89 (2005)
Sun, X. et al. Activation of integrin α5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc. Natl Acad. Sci. USA 113, 769–774 (2016)
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007)
Varelas, X. et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nature Cell Biol. 10, 837–848 (2008)
Takagi, J., Petre, B. M., Walz, T. & Springer, T. A. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–611 (2002)
Luo, J. et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nature Protocols 2, 1236–1247 (2007)
Stemmer, M., Thumberger, T., Del Sol Keyer, M., Wittbrodt, J. & Mateo, J. L. CCTop: An intuitive, flexible and reliable CRISPR/Cas9 target prediction Tool. PLoS ONE 10, e0124633 (2015)
Varadi, K. et al. Novel random peptide libraries displayed on AAV serotype 9 for selection of endothelial cell-directed gene transfer vectors. Gene Ther. 19, 800–809 (2012)
Grieger, J. C., Choi, V. W. & Samulski, R. J. Production and characterization of adeno-associated viral vectors. Nature Protocols 1, 1412–1428 (2006)
Acknowledgements
We thank S. Chien for commenting on the manuscript, and C.-I. Lee and T.-E. Lin for conducting clip experiments. This study was supported by the Hong Kong Research Grants Council (CUHK2/CRF/12G), Natural Science Foundation of China (91339117, 81130002, 31430045), RGC (T12-402/13-N, C7055-14G, CUHK14105814), Croucher Foundation, CUHK Vice Chancellor’s Discretionary Fund, Lui Che Woo Foundation, and the Ministry of Science and Technology, Taiwan (MOST104-2321-B-400-017, MOST104-2320-B-400-002-MY3).
Author information
Authors and Affiliations
Contributions
L.W. designed the study, conducted most experiments, analysed the data and wrote the manuscript; J.Y.L. helped western blot, contributed ideas and prepared the manuscript. X.Y.T. helped revise the manuscript and provided suggestions for disturbed-flow-induced atherosclerosis; B.L. and D.A. generated EC-specific YAP transgenic mice and performed the in vivo study. Y.H.H. and D.D. performed plasmid construction. L.J.C., J.L. and C.W.L. performed immunohistochemistry and the carotid artery partial ligation surgery. J.J.C. and S.W. helped with atherosclerotic samples and contributed to data analysis. K.L.M., K.K.T. and K.M.K. helped in animal studies. J.J.C. and N.W. provided constructive suggestions in experimental design and helped revise the manuscript. Y.Z. and Y.H. are the leading principal investigators who directed the study and data analysis, and prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information
Nature thanks P. F. Davies, G. Halder and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
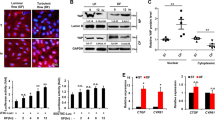
Extended Data Figure 1 USS and disturbed flow oppositely regulate YAP/TAZ activity.
a, Immunoblotting showing USS induces YAP phosphorylation in human aortic ECs. b, Summarized data for USS-induced YAP nuclear exportation (n = 5; *P < 0.05 by two-tailed unpaired t-test). c, TAZ is decreased in nuclear fractions and increased in cytoplasmic fractions in HUVECs exposed to USS for 6 h. TAZ expression was detected by immunoblotting after cell fractionation. d, Disturbed flow suppresses YAP phosphorylation in human aortic ECs. e, Immunoblotting showing disturbed flow increases CTGF expression in HUVECs. All immunoblotting experiments were repeated three times and the representative results are shown. f, g, YAP/TAZ knockdown attenuates gene expression of disturbed-flow-induced (f) CTGF and (g) CYR61 (n = 3; *P < 0.05 by two-tailed unpaired t-test). NS, not significant. h, Summarized data for en face staining of relative nuclear YAP level in mouse aorta (nTA = 6, nAA, inner = 3, nAA, outer = 3; *P < 0.05 by two-tailed unpaired t-test).
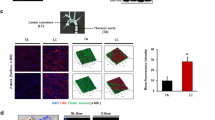
Extended Data Figure 2 USS inhibits YAP/TAZ through integrin–Gα13–RhoA pathway.
a, MnCl2 (0.5 mM) promotes YAP phosphorylation shown by immunoblotting. b, MnCl2 reduces nuclear YAP/TAZ levels in HUVECs. c, Gα13 inhibiting peptide mSRI reverses MnCl2-induced YAP/TAZ reporter (8×GTIIC-luc) gene activity (n = 3; *P < 0.05 by two-tailed unpaired t-test). d, RGD-containing peptide GRGDSP downregulates YAP/TAZ downstream target gene expression (n = 3; *P < 0.05 by two-tailed unpaired t-test). e, f, Pro32pro33 mutation in integrin β3 inhibits YAP/TAZ transactivation in HUVECs, as verified by suppressed (e) expression of YAP/TAZ target genes and (f) YAP/TAZ reporter gene activity (n = 3; *P < 0.05 by two-tailed unpaired t-test). g, Gα13 or integrin β3 knockdown reverses MnCl2-induced YAP/TAZ nuclear exportation in HUVECs. h, Gα13 knockdown reverses RGD-containing peptide-mediated CTGF and CYR61 suppression in HUVECs (n = 3; *P < 0.05 by two-tailed unpaired t-test). i, Gα13 inhibiting peptide mSRI and mP6 reverse MnCl2-induced (5 min) pYAP but not total YAP expression in HUVECs. The experiments were repeated at least three times and the representative results are shown.
Extended Data Figure 3 YAP/TAZ activation increases JNK activity.
a, Heatmap for mRNA sequencing results showing CA-YAP/TAZ promotes expression of pro-inflammatory genes. b, CA-YAP/TAZ increases the promoter activity of adhesion molecules in HUVECs (n = 4; *P < 0.05 by two-tailed unpaired t-test). c, Summarized data for CA-YAP/TAZ overexpression increases monocyte attachment to HUVECs (n = 4; *P < 0.05 by two-tailed unpaired t-test). d, e, Immunoblotting showing JNK phosphorylation in HUVECs exposed to (d) USS or (e) disturbed flow for different durations. Experiments were repeated three times and the representative results are shown. f, YAP/TAZ knockdown suppresses basal and PMA-induced JNK phosphorylation in HUVECs. g, Overexpression of dominant-negative YAP (YAP S94A) inhibits PMA-induced AP-1 reporter gene activity (n = 3; *P < 0.05 by two-tailed unpaired t-test). h, CA-YAP/TAZ increases AP-1 reporter gene activity in HUVECs (n = 4; *P < 0.05 by two-tailed unpaired t-test), and PMA was used as positive control for monitoring AP-1 activity.
Extended Data Figure 4 EC-specific overexpression of YAP accelerates plaque formation.
a, The generation of Cre-mediated EC-specific YAP overexpression transgenic mice. b, En face staining showing increased YAP expression in endothelial cells of the Tie2Cre/+;Yap-COEtg/+;ApoE−/− (n = 10). c, Summarized data for EC-specific YAP overexpression-increased JNK phosphorylation (n = 10; *P < 0.05 by two-tailed unpaired t-test). d, EC-specific YAP overexpression increases macrophage content in the atherosclerotic plaques from aortic root (n = 10; *P < 0.05 by two-tailed unpaired t-test). e, f, EC-specific YAP overexpression does not affect serum levels of (e) cholesterol or (f) triglycerides (n = 10; *P < 0.05 by two-tailed unpaired t-test).
Extended Data Figure 5 Inhibiting TAZ activity by shRNA or MnCl2 administration delays atherogenesis and is independent of lipid metabolism, while activating YAP/TAZ by AAV-mediated CA-YAP/TAZ overexpression accelerates atherosclerotic plaque formation.
a, Immunoblotting showing adenovirus-mediated TAZ shRNA suppressed TAZ expression level. b, TAZ knockdown delayed Western-diet-induced plaque formation in ApoE−/− mice, n = 5; *P < 0.05 by two-tailed unpaired t-test. c, TAZ knockdown-suppressed plaque formation in ApoE−/− mice is not due to change in lipid profile. Data are expressed as mean ± s.e.m., n = 5; *P < 0.05 by two-tailed unpaired t-test. d, Immunoblotting showing increased YAP expression in mice injected with AAV expressing CA-YAP/TAZ. e, f, Oil Red O staining (e) and summarized data (f) for CA-YAP/TAZ-induced exacerbation of plaque formation; n = 5, *P < 0.05 by two-tailed unpaired t-test. g. AAV-mediated CA-YAP/TAZ overexpression does not affect lipid profile in ApoE−/− mice. h, i, Oral administration of MnCl2 does not affect (h) lipid profile or (i) SOD activity in liver. Data are expressed as mean ± s.e.m., n = 5; *P < 0.05 by two-tailed unpaired t-test.
Extended Data Figure 6 Summary of western blotting data.
a, Endothelium removal reduces YAP level in mouse aorta. b, USS increases YAP phosphorylation. c, Disturbed flow reduces YAP phosphorylation. d, Thoracic aorta expresses higher levels of pYAP than aortic arch. e, Overexpression loss-of-function mutation of integrin β3 (β3Δcyto) suppresses USS-induced pYAP. f, RGD-containing peptide GRGDSP induces pYAP. g, Gα13 or integrin β3 knockdown reverses MnCl2-induced pYAP. h, Integrin gain-of-function mutation Pro32Pro33 increases pYAP. i, Constitutively activated RhoA (CA-RhoA) reverses USS-induced pYAP. Data: n = 6 for a and n = 3 for other figures; *P < 0.05 by two-tailed unpaired t-test.
Extended Data Figure 7 Summary of western blotting data.
a, CA-RhoA reverses MnCl2-induced pYAP. b, Gα13 knockdown reverses USS-induced pYAP. c, Gα13 inhibitor SRI reverses USS-induced pYAP. d–h, Immunoblotting detection of (d) pYAP, (e) YAP, (f) TAZ, (g) Gα13 and (h) integrin β3 levels. i, YAP knockdown by the CRISPR-Cas9 in vivo genome editing system. Data: n = 3 for a–c, n = 5 for d–i; *P < 0.05 by two-tailed unpaired t-test.
Supplementary information
Supplementary Information
This file contains Supplementary Figure 1, the original western blot scans and Supplementary Table 1, the information for primers used in the study. (PDF 1477 kb)
Source data
Rights and permissions
About this article
Cite this article
Wang, L., Luo, JY., Li, B. et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540, 579–582 (2016). https://doi.org/10.1038/nature20602
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20602
This article is cited by
-
Biomaterial-based mechanical regulation facilitates scarless wound healing with functional skin appendage regeneration
Military Medical Research (2024)
-
Mitochondrial stress activates YAP/TAZ through RhoA oxidation to promote liver injury
Cell Death & Disease (2024)
-
VPS35 promotes gastric cancer progression through integrin/FAK/SRC signalling-mediated IL-6/STAT3 pathway activation in a YAP-dependent manner
Oncogene (2024)
-
Validation of Signal Intensity Gradient from TOF-MRA for Wall Shear Stress by Phase-Contrast MR
Journal of Imaging Informatics in Medicine (2024)
-
Integrin signaling in cancer: bidirectional mechanisms and therapeutic opportunities
Cell Communication and Signaling (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.