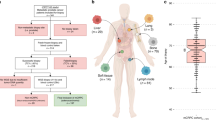
Abstract
Germ-cell tumours (GCTs) are derived from germ cells and occur most frequently in the testes1,2. GCTs are histologically heterogeneous and distinctly curable with chemotherapy3. Gains of chromosome arm 12p and aneuploidy are nearly universal in GCTs4,5,6, but specific somatic genomic features driving tumour initiation, chemosensitivity and progression are incompletely characterized. Here, using clinical whole-exome and transcriptome sequencing of precursor, primary (testicular and mediastinal) and chemoresistant metastatic human GCTs, we show that the primary somatic feature of GCTs is highly recurrent chromosome arm level amplifications and reciprocal deletions (reciprocal loss of heterozygosity), variations that are significantly enriched in GCTs compared to 19 other cancer types. These tumours also acquire KRAS mutations during the development from precursor to primary disease, and primary testicular GCTs (TGCTs) are uniformly wild type for TP53. In addition, by functional measurement of apoptotic signalling (BH3 profiling) of fresh tumour and adjacent tissue7, we find that primary TGCTs have high mitochondrial priming that facilitates chemotherapy-induced apoptosis. Finally, by phylogenetic analysis of serial TGCTs that emerge with chemotherapy resistance, we show how TGCTs gain additional reciprocal loss of heterozygosity and that this is associated with loss of pluripotency markers (NANOG and POU5F1)8,9 in chemoresistant teratomas or transformed carcinomas. Our results demonstrate the distinct genomic features underlying the origins of this disease and associated with the chemosensitivity phenotype, as well as the rare progression to chemoresistance. These results identify the convergence of cancer genomics, mitochondrial priming and GCT evolution, and may provide insights into chemosensitivity and resistance in other cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Oosterhuis, J. W. & Looijenga, L. H. Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 5, 210–222 (2005)
Sonne, S. B. et al. Analysis of gene expression profiles of microdissected cell populations indicates that testicular carcinoma in situ is an arrested gonocyte. Cancer Res. 69, 5241–5250 (2009)
Hanna, N. H. & Einhorn, L. H. Testicular cancer—discoveries and updates. N. Engl. J. Med. 371, 2005–2016 (2014)
Atkin, N. B. & Baker, M. C. Specific chromosome change, i(12p), in testicular tumours? Lancet 2, 1349 (1982)
Litchfield, K. et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat. Commun. 6, 5973 (2015)
Oosterhuis, J. W. et al. Ploidy of primary germ cell tumors of the testis. Pathogenetic and clinical relevance. Lab. Invest. 60, 14–21 (1989)
Montero, J. et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell 160, 977–989 (2015)
Looijenga, L. H. et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 63, 2244–2250 (2003)
Hart, A. H. et al. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer 104, 2092–2098 (2005)
Moch, H., Cubilla, A. L., Humphrey, P. A., Reuter, V. E. & Ulbright, T. M. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur. Urol. 70, 93–105 (2016)
Gutekunst, M. et al. Cisplatin hypersensitivity of testicular germ cell tumors is determined by high constitutive Noxa levels mediated by Oct-4. Cancer Res. 73, 1460–1469 (2013)
Jacobsen, C. & Honecker, F. Cisplatin resistance in germ cell tumours: models and mechanisms. Andrology 3, 111–121 (2015)
Rijlaarsdam, M. A. & Looijenga, L. H. An oncofetal and developmental perspective on testicular germ cell cancer. Semin. Cancer Biol. 29, 59–74 (2014)
Rapley, E. A. et al. A genome-wide association study of testicular germ cell tumor. Nat. Genet. 41, 807–810 (2009)
Kanetsky, P. A. et al. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat. Genet. 41, 811–815 (2009)
Feldman, D. R. et al. Presence of somatic mutations within PIK3CA, AKT, RAS, and FGFR3 but not BRAF in cisplatin-resistant germ cell tumors. Clinical Cancer Res. 20, 3712–3720 (2014)
Stachler, M. D. et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nat. Genet. 47, 1047–1055 (2015)
Brastianos, P. K. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 5, 1164–1177 (2015)
Lawrence, M. S. et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014)
Goudarzi, K. M. & Lindström, M. S. Role of ribosomal protein mutations in tumor development (Review). Int. J. Oncol. 48, 1313–1324 (2016)
Wang, L. et al. Novel somatic and germline mutations in intracranial germ cell tumours. Nature 511, 241–245 (2014)
Carter, S. L. et al. Absolute quantification of somatic DNA alterations in human cancer. Nat. Biotechnol. 30, 413–421 (2012)
Zack, T. I. et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140 (2013)
Baker, D. E. et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat. Biotechnol. 25, 207–215 (2007)
Närvä, E. et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat. Biotechnol. 28, 371–377 (2010)
Liu, J. C. et al. High mitochondrial priming sensitizes hESCs to DNA-damage-induced apoptosis. Cell Stem Cell 13, 483–491 (2013)
Ni Chonghaile, T. et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334, 1129–1133 (2011)
Abada, P. B. & Howell, S. B. Cisplatin induces resistance by triggering differentiation of testicular embryonal carcinoma cells. PLoS One 9, e87444 (2014)
Wermann, H. et al. Global DNA methylation in fetal human germ cells and germ cell tumours: association with differentiation and cisplatin resistance. J. Pathol. 221, 433–442 (2010)
Jørgensen, A. et al. Dysregulation of the mitosis-meiosis switch in testicular carcinoma in situ. J. Pathol. 229, 588–598 (2013)
Hoffman, H. violin.m — Simple violin plot using Matlab default kernel estimation. INRES (Univ. of Bonn, 2015)
Van Allen, E. M. et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat. Med. 20, 682–688 (2014)
Cibulskis, K. et al. ContEst: estimating cross-contamination of human samples in next-generation sequencing data. Bioinformatics 27, 2601–2602 (2011)
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213–219 (2013)
Saunders, C. T. et al. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28, 1811–1817 (2012)
Costello, M. et al. Discovery and characterization of artifactual mutations in deep coverage targeted capture sequencing data due to oxidative DNA damage during sample preparation. Nucleic Acids Res. 41, e67 (2013)
Ramos, A. H. et al. Oncotator: cancer variant annotation tool. Hum. Mutat. 36, E2423–E2429 (2015)
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013)
Thorvaldsdóttir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 (2013)
Olshen, A. B., Venkatraman, E. S., Lucito, R. & Wigler, M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics 5, 557–572 (2004)
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009)
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011)
Touzeau, C. et al. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia 30, 761–764 (2016)
Acknowledgements
We thank the patients for contributing to this study, and H.Taylor-Weiner for feedback on ES cells. This work was supported by NIH U54 HG003067, NIH 1K08 CA188615 (E.M.V.), Damon Runyon Clinical Investigator Award (E.M.V.), Shawmut Design and Construction Pan Mass Challenge Team (C.S.), and Giovino Jimmy Fund Golf Tournament (C.S.).
Author information
Authors and Affiliations
Contributions
A.T.-W., T.Z., B.B., G.C.H., S.A., A.A.-M. and E.M.V. performed genomic analysis of discovery cohort. A.T.-W., T.Z., B.B., E.O., M.H., C.S. and E.M.V performed clinical integration and analysis. J.L.G. and A.L. performed BH3 profiling experiments. S.S., S.L.C., R.B. and G.G. contributed methodology and analysis review. A.R. and E.M.V. performed biological review of genomic findings. S.G. performed sequencing assays. A.T.-W., T.Z., K.L., C.T. and E.M.V. performed genomic analysis of validation cohort. M.H. performed pathology and histological evaluation of clinical samples. A.T.-W., T.Z., B.B., C.S. and E.M.V. prepared manuscript and figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks K. Nathanson and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Mutational significance and copy number meta-analysis.
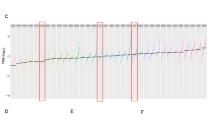
a, Mutational significance meta-analysis of discovery and ICR cohorts identify KRAS, KIT and RPL5 as significantly mutated in TGCT, with a spectrum of mutation rates. In this plot, each column represents a patient WES. Asterisk denotes the hypermutated PMGCT (DFCI_17). b, RLOH distribution by histology in discovery cohort. c, RLOH distribution by histology in ICR cohort. d, RLOH distribution in the meta-analysis, consistent with both subsets.
Extended Data Figure 2 Genomic reads for KRAS loci in two patient cases.
a, Integrative genomics viewer snapshot of KRAS p.G12A mutation in DFCI_55 GCNIS and seminoma. The mutation is present in the primary tumour but absent from the GCNIS. b, Integrative genomics viewer snapshot of KRAS p.G12A mutation in DFCI_61 GCNIS and seminoma. The mutation is present in the primary tumour but absent from the GCNIS.
Extended Data Figure 3 Allelic copy number heat map of the discovery cohort.
Each tumour sample is a row, and chromosomes are listed as columns. Blue regions note deletions, and red regions denote amplifications.
Extended Data Figure 4 Testes tumours of different cell types.
Allelic copy number data from testes tumours of different cell types are shown. These three tumours do not contain the same level of arm level chromosomal events as GCTs.
Extended Data Figure 5 Phylogenetic analysis of DFCI_4.
Histology proportion is indicated by pie charts within each phylogenetic tree. Phylogenetic trees were constructed using allelic copy number deconstructions. Branch lengths are proportional to the number of deconstructed copy number events. Branches leading to primary samples are red, and branches leading to metastases are purple. The dotted branch indicates deconstructions which may be impacted by FFPE sample degradation, limiting discrete branch length estimation.
Supplementary information
Supplementary Table 1
Clinical and genomic overview of GCT cohort. This table lists histological subclass, vital status, mutation load, and location of the primary and initially sequenced metastases shown in figure 1. (XLSX 45 kb)
Supplementary Table 2
Summary clinical data. This table lists aggregate summary phenotypic data, including therapies and response, for this cohort. (XLSX 10 kb)
Supplementary Table 3
Mutation significance analysis. Table of significant (q < 0.2) genes uncovered with MutSigCV run on the discovery cohort. (XLSX 34 kb)
Supplementary Table 4
Mutation data for all samples. All mutations and small insertions and deletions called in this cohort. (XLSX 486 kb)
Supplementary Table 5
ABSOLUTE allelic segmented copy-number data. Allelic copy number data used to perform deconstructions and construct phylogenetic trees. (TXT 1236 kb)
Supplementary Table 6
ABSOLUTE purity and ploidy solutions. This table lists purity, ploidy and genome doubling status of each tumor as assessed by ABSOLUTE. (XLS 21 kb)
Supplementary Table 7
Gene expression data. This table has transcript per million expression values by sample for TP53, NANOG and POU5F1. (XLSX 46 kb)
Supplementary Table 8
Detailed clinical annotations for multi-regional sampling subset. This table lists treatment regimen, location, and histological subtype of each sample in figure 4. (XLSX 36 kb)
Source data
Rights and permissions
About this article
Cite this article
Taylor-Weiner, A., Zack, T., O’Donnell, E. et al. Genomic evolution and chemoresistance in germ-cell tumours. Nature 540, 114–118 (2016). https://doi.org/10.1038/nature20596
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20596
This article is cited by
-
Evolutionary determinants of curability in cancer
Nature Ecology & Evolution (2023)
-
Integrated genomic analysis reveals aberrations in WNT signaling in germ cell tumors of childhood and adolescence
Nature Communications (2023)
-
RANKL regulates testicular cancer growth and Denosumab treatment has suppressive effects on GCNIS and advanced seminoma
British Journal of Cancer (2022)
-
Hodentumoren aus klinischer Sicht
Die Pathologie (2022)
-
Clonal diversification and histogenesis of malignant germ cell tumours
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.