Abstract
Receptor-interacting protein kinase 1 (RIPK1) regulates cell death and inflammation through kinase-dependent and -independent functions1,2,3,4,5,6,7. RIPK1 kinase activity induces caspase-8-dependent apoptosis and RIPK3 and mixed lineage kinase like (MLKL)-dependent necroptosis8,9,10,11,12,13. In addition, RIPK1 inhibits apoptosis and necroptosis through kinase-independent functions, which are important for late embryonic development and the prevention of inflammation in epithelial barriers14,15,16,17,18. The mechanism by which RIPK1 counteracts RIPK3–MLKL-mediated necroptosis has remained unknown. Here we show that RIPK1 prevents skin inflammation by inhibiting activation of RIPK3–MLKL-dependent necroptosis mediated by Z-DNA binding protein 1 (ZBP1, also known as DAI or DLM1). ZBP1 deficiency inhibited keratinocyte necroptosis and skin inflammation in mice with epidermis-specific RIPK1 knockout. Moreover, mutation of the conserved RIP homotypic interaction motif (RHIM) of endogenous mouse RIPK1 (RIPK1mRHIM) caused perinatal lethality that was prevented by RIPK3, MLKL or ZBP1 deficiency. Furthermore, mice expressing only RIPK1mRHIM in keratinocytes developed skin inflammation that was abrogated by MLKL or ZBP1 deficiency. Mechanistically, ZBP1 interacted strongly with phosphorylated RIPK3 in cells expressing RIPK1mRHIM, suggesting that the RIPK1 RHIM prevents ZBP1 from binding and activating RIPK3. Collectively, these results show that RIPK1 prevents perinatal death as well as skin inflammation in adult mice by inhibiting ZBP1-induced necroptosis. Furthermore, these findings identify ZBP1 as a critical mediator of inflammation beyond its previously known role in antiviral defence and suggest that ZBP1 might be implicated in the pathogenesis of necroptosis-associated inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Christofferson, D. E., Li, Y. & Yuan, J. Control of life-or-death decisions by RIP1 kinase. Annu. Rev. Physiol. 76, 129–150 (2014)
Newton, K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 25, 347–353 (2015)
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015)
Silke, J., Rickard, J. A. & Gerlic, M. The diverse role of RIP kinases in necroptosis and inflammation. Nat. Immunol. 16, 689–697 (2015)
Weinlich, R. & Green, D. R. The two faces of receptor interacting protein kinase-1. Mol. Cell 56, 469–480 (2014)
Chan, F. K., Luz, N. F. & Moriwaki, K. Programmed necrosis in the cross talk of cell death and inflammation. Annu. Rev. Immunol. 33, 79–106 (2015)
Lukens, J. R. et al. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature 498, 224–227 (2013)
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 (2005)
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009)
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008)
Berger, S. B. et al. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 192, 5476–5480 (2014)
Polykratis, A. et al. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 193, 1539–1543 (2014)
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009)
Dannappel, M. et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513, 90–94 (2014)
Dillon, C. P. et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 157, 1189–1202 (2014)
Kaiser, W. J. et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc. Natl Acad. Sci. USA 111, 7753–7758 (2014)
Rickard, J. A. et al. RIPK1 regulates RIPK3–MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157, 1175–1188 (2014)
Takahashi, N. et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 513, 95–99 (2014)
Kaiser, W. J., Upton, J. W. & Mocarski, E. S. Receptor-interacting protein homotypic interaction motif-dependent control of NF-κB activation via the DNA-dependent activator of IFN regulatory factors. J. Immunol. 181, 6427–6434 (2008)
Rebsamen, M. et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-κB. EMBO Rep. 10, 916–922 (2009)
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007)
Schwartz, T., Behlke, J., Lowenhaupt, K., Heinemann, U. & Rich, A. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat. Struct. Biol. 8, 761–765 (2001)
Fu, Y. et al. Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene 240, 157–163 (1999)
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11, 290–297 (2012)
Ishii, K. J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008)
Bonnet, M. C. et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35, 572–582 (2011)
Kelliher, M. A. et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 (1998)
Meng, L., Jin, W. & Wang, X. RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc. Natl Acad. Sci. USA 112, 11007–11012 (2015)
Kuriakose, T. et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 1, aag2045–aag2045 (2016)
Hafner, M. et al. Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis 38, 176–181 (2004)
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004)
Repetto, G., del Peso, A. & Zurita, J. L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protocols 3, 1125–1131 (2008)
Acknowledgements
We thank Genentech and V. Dixit for Ripk3−/− mice, S. Akira for Zbp1−/− mice, and J. Han for lentiviral vectors. We thank B. Zevnik, P. Jankowski and S. Assenmacher at the CECAD Transgenic Core Facility for CRISPR/Cas9 mutagenesis in mouse zygotes and C. Uthoff-Hachenberg, J. Buchholz, E. Mahlberg and B. Kühnel for excellent technical assistance. Research reported in this publication was supported by funding from the ERC (grant agreement no. 323040) and the DFG (SFB829 and SFB670). J.L. was supported by a Humboldt research fellowship and C.K. was supported by a Humboldt research fellowship and an EMBO long-term fellowship.
Author information
Authors and Affiliations
Contributions
J.L. designed and generated the Ripk1mRHIM mice. J.L. and C.K. analysed Ripk1mRHIM/mRHIM and RIPK1mRHIM/E-KO mice and performed genetic crosses to address the role of RIPK3 and ZBP1 in these mice. J.L. and C.K. carried out all immunoblots and immunoprecipitation experiments. S.K. generated and characterized RIPK1E-KO Zbp1−/− and FADDE-KO Zbp1−/− mice and made the initial discovery that ZBP1 is required for keratinocyte necroptosis in RIPK1E-KO mice. S.K. and T-M.V. conducted immunostainings and qRT–PCR assays in skin samples from RIPK1E-KO and RIPK1mRHIM/E-KO mice. A.P. generated the Ripk1fl/fl mice and L.W. generated Mlkl−/− mice. M.P. supervised the study, interpreted data and wrote the manuscript together with J.L., C.K. and S.K. J.L., C.K. and S.K. contributed equally and their order of appearance in the author list is random.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks H. Walczak and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
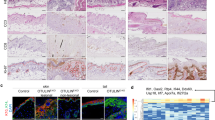
Extended Data Figure 1 ZBP1 deficiency strongly delays and ameliorates skin inflammation in RIPK1E-KO mice.
a, Photographs of mice with the indicated genotypes at the age of 4 weeks. Images shown are representative of n ≥ 60 RIPK1E-KO and n ≥ 40 RIPK1E-KO Zbp1−/− mice. b, Photographs of mice with the indicated genotypes and age. Images shown are representative of n ≥ 4 RIPK1E-KO mice at the age of 5–7 weeks and n ≥ 20 RIP1E-KO Zbp1−/− mice at the age of 17–35 weeks. c, Table summarizing the macroscopically observed skin lesions and time of sacrifice of 21 aged RIPK1E-KO Zbp1−/− mice. d, Representative images of skin sections from RIPK1E-KO Zbp1−/− mice and their respective controls stained with H&E (n ≥ 18) or immunostained with the indicated antibodies (n ≥ 4) at the age of 17-35 weeks. Nuclei stained with DAPI. Scale bars, 50 μm. e, Representative images of skin sections from 4–5 week old RIPK1E-KO (n ≥ 6) and RIPK1E-KO Zbp1−/− (n ≥ 3) and their respective control mice stained with TUNEL or immunostained with anti-CC3 antibodies. Nuclei stained with DAPI. Scale bars, 50 μm. f, Microscopic quantification of CC3 and TUNEL positive cells on skin sections from 4–5 week old mice with the indicated genotypes. Epi, epidermis; der, dermis. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005.
Extended Data Figure 2 ZBP1 is not required for keratinocyte necroptosis and skin inflammation in mice with epidermis-specific FADD deficiency.
a, Representative photographs depicting the macroscopically observed phenotype of FADDE-KO (n ≥ 10) and FADDE-KO Zbp1−/− (n = 5) mice at the age of 6 days. b, Representative images of skin sections from 6 day old mice with the indicated genotypes stained with H&E (n = 6) or immunostained with the indicated antibodies (n = 3). Nuclei stained with DAPI. Scale bars, 50 μm. c, qRT–PCR analysis of the mRNA expression of the indicated cytokines and chemokines in total skin RNA from 6-day-old mice with the indicated genotypes. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005.
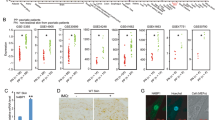
Extended Data Figure 3 CRISPR–Cas9-mediated generation of Ripk1mRHIM and Mlkl−/− mice.
a, Schematic depiction of the generation of Ripk1mRHIM/mRHIM mice indicating the sequence of the sgRNA and the single stranded oligonucleotide used for mutating the RHIM domain that were introduced by pronuclear injection into mouse zygotes and the sequencing result of one of the two obtained founders. b, Small intestinal sections from E18.5 pups were stained with H&E or TUNEL or immunostained with anti-CC3 antibodies. Representative images shown (wild type n = 6 for H&E, n = 3 for TUNEL and anti-CC3; Ripk1mRHIM/mRHIM n = 5 for H&E, n = 3 for TUNEL and anti-CC3; Ripk1−/− n = 3 for H&E, TUNEL and anti-CC3). Nuclei stained with DAPI. Scale bars, 50 μm. c, Microscopic quantification of CC3+ and TUNEL+ cells on gut sections from E18.5 pups with the indicated genotypes. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005. d, Diagram indicating the sgRNA target sequence (capital letters) used to generate a mutation in exon 2 downstream of the ATG of the Mlkl gene. The PAM sequence is indicated in red. Sequencing showing the 2 base pair deletion found in 97 at position chr8:111,333,648–111,333,649 (mm10), which results in a frameshift after amino acid 34 and a premature stop codon at amino acid position 55 of MLKL. This Mlkl knockout allele was used throughout this study.
Extended Data Figure 4 Rescue of perinatal lethality of Ripk1mRHIM/mRHIM mice by deficiency of ZBP1, MLKL or RIPK3.
a, Representative photographs and body weights of the indicated mice. b, Representative H&E stainings of skin, liver, spleen, colon and small intestine sections from 5-month-old Ripk3WT/−(n = 4), Ripk1mRHIM/mRHIMRipk3−/− (n = 4) and Ripk1mRHIM/mRHIMZbp1−/− mice (n = 3).
Extended Data Figure 5 MLKL or ZBP1-deficiency prevents skin inflammation in RIPK1mRHIM/E-KO mice.
a, Representative photographs of RIPK1mRHIM/E-KO (n = 9), RIPK1mRHIM/E-KO MlklWT/− (n = 11), RIPK1mRHIM/E-KO Mlkl−/− (n = 16) and RIPK1mRHIM/E-KO Zbp1−/− (n = 7) at the age of 9–11 weeks. b, Representative images of skin sections from 9–11-week-old RIPK1mRHIM/E-KO MlklWT/− (n = 11) and the respective control mice stained with H&E or immunostained with the indicated antibodies. Nuclei stained with DAPI. Scale bars, 50 μm. c, Representative images of skin sections from 9–11-week-old RIPK1mRHIM/E-KO (n ≥ 6), RIPK1mRHIM/E-KO Mlkl−/− (n = 3), RIPK1mRHIM/E-KO Zbp1−/− (n = 3) and their respective control mice stained with TUNEL or immunostained with anti-CC3 antibodies. Nuclei stained with DAPI. Scale bars, 50 μm. d, Microscopic quantification of CC3+ and TUNEL+ positive cells on skin sections from 9–11 week old mice with the indicated genotypes. Epi, epidermis; der, dermis. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005.
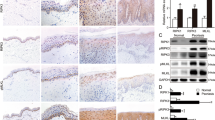
Extended Data Figure 6 Expression of ZBP1 in primary keratinocytes.
a, Immunoblot analysis of lysates of primary keratinocytes derived from mice with the indicated genotypes. Each lane represents keratinocytes from individual mice. Cell lysates of wild-type FLM was used as a positive control. For gel source data, see Supplementary Fig. 1. b, Immunoblot analysis of primary keratinocytes derived from mice with the indicated genotypes were left untreated (‘medium’) or stimulated with TNF or IFN-β for 18 h. For gel source data, see Supplementary Fig. 1.
Supplementary information
Supplementary Information
This file contains the uncropped blot scans. (PDF 852 kb)
Rights and permissions
About this article
Cite this article
Lin, J., Kumari, S., Kim, C. et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540, 124–128 (2016). https://doi.org/10.1038/nature20558
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20558
This article is cited by
-
Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity
Nature Reviews Cancer (2024)
-
Cytosolic DNA sensors in neurodegenerative diseases: from physiological defenders to pathological culprits
EMBO Molecular Medicine (2024)
-
Saracatinib inhibits necroptosis and ameliorates psoriatic inflammation by targeting MLKL
Cell Death & Disease (2024)
-
Global trends in PANoptosis research: bibliometrics and knowledge graph analysis
Apoptosis (2024)
-
MLKL post-translational modifications: road signs to infection, inflammation and unknown destinations
Cell Death & Differentiation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.