Abstract
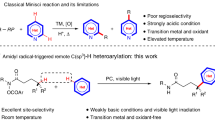
Despite advances in hydrogen atom transfer (HAT) catalysis1,2,3,4,5, there are currently no molecular HAT catalysts that are capable of homolysing the strong nitrogen–hydrogen (N–H) bonds of N-alkyl amides. The motivation to develop amide homolysis protocols stems from the utility of the resultant amidyl radicals, which are involved in various synthetically useful transformations, including olefin amination6,7,8,9,10,11 and directed carbon–hydrogen (C–H) bond functionalization12,13,14,15,16. In the latter process—a subset of the classical Hofmann–Löffler–Freytag reaction—amidyl radicals remove hydrogen atoms from unactivated aliphatic C–H bonds17,18,19,20,21. Although powerful, these transformations typically require oxidative N-prefunctionalization of the amide starting materials to achieve efficient amidyl generation. Moreover, because these N-activating groups are often incorporated into the final products, these methods are generally not amenable to the direct construction of carbon–carbon (C–C) bonds. Here we report an approach that overcomes these limitations by homolysing the N–H bonds of N-alkyl amides via proton-coupled electron transfer. In this protocol, an excited-state iridium photocatalyst and a weak phosphate base cooperatively serve to remove both a proton and an electron from an amide substrate in a concerted elementary step. The resultant amidyl radical intermediates are shown to promote subsequent C–H abstraction and radical alkylation steps. This C–H alkylation represents a catalytic variant of the Hofmann–Löffler–Freytag reaction, using simple, unfunctionalized amides to direct the formation of new C–C bonds. Given the prevalence of amides in pharmaceuticals and natural products, we anticipate that this method will simplify the synthesis and structural elaboration of amine-containing targets. Moreover, this study demonstrates that concerted proton-coupled electron transfer can enable homolytic activation of common organic functional groups that are energetically inaccessible using traditional HAT-based approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mayer, J. M. Understanding hydrogen atom transfer: from bond strengths to Marcus theory. Acc. Chem. Res. 44, 36–46 (2011)
Liu, W. et al. Oxidative aliphatic C–H fluorination with fluroide ion catalyzed by a manganese porphyrin. Science 337, 1322–1325 (2012)
Jeffrey, J. L., Terrett, J. A. & MacMillan, D. W. C. O–H hydrogen bonding promotes H-atom transfer from α C–H bonds for C-alkylation of alcohols. Science 349, 1532–1536 (2015)
Chen, M. S. & White, M. C. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science 327, 566–571 (2010)
Newhouse, T. & Baran, P. S. If C–H bonds could talk: selective C–H bond oxidation. Angew. Chem. Int. Ed. 50, 3362–3374 (2011)
Choi, G. J. & Knowles, R. R. Catalytic alkene carboaminations enabled by oxidative proton-coupled electron transfer. J. Am. Chem. Soc. 137, 9226–9229 (2015)
Miller, D. C., Choi, G. J., Orbe, H. S. & Knowles, R. R. Catalytic olefin hydroamidation enabled by proton-coupled electron transfer. J. Am. Chem. Soc. 137, 13492–13495 (2015)
Li, Z., Song, L. & Li, C. Silver-catalyzed radical aminofluorination of unactivated alkenes in aqueous media. J. Am. Chem. Soc. 135, 4640–4643 (2013)
Kemper, J. & Studer, A. Stable reagents for the generation of N-centered radicals: hydroamination of norbornene. Angew. Chem. Int. Ed. 44, 4914–4917 (2005)
Guin, J., Fröhlich, R. & Studer, A. Thiol-catalyzed stereoselective transfer hydroamination of olefins with N-aminated dihydropyridines. Angew. Chem. Int. Ed. 47, 779–782 (2008)
Zard, S. Z. Recent progress in the generation and use of nitrogen-centered radicals. Chem. Soc. Rev. 37, 1603–1618 (2008)
Martín, A., Pérez-Martín, I. & Suárez, E. Intramolecular hydrogen abstraction promoted by amidyl radicals. Evidence for electronic factors in the nucleophilic cyclization of ambident amides to oxocarbenium ions. Org. Lett. 7, 2027–2030 (2005)
Chen, K., Richter, J. M. & Baran, P. S. 1,3-Diol synthesis via controlled, radical-mediated C–H functionalization. J. Am. Chem. Soc. 130, 7247–7249 (2008)
Schmidt, V. A., Quinn, R. K., Bruscoe, A. T. & Alexanian, E. J. Site-selective aliphatic C–H bromination using N-bromoamides and visible light. J. Am. Chem. Soc. 136, 14389–14392 (2014)
Liu, T., Mei, T.-S. & Yu, J.-Q. γ,δ,ε-C(sp3)–H functionalization through directed radical H-abstraction. J. Am. Chem. Soc. 137, 5871–5874 (2015)
Horner, J. H., Musa, O. M., Bouvier, A. & Newcomb, M. Absolute kinetics of amidyl radical reactions. J. Am. Chem. Soc. 120, 7738–7748 (1998)
Topczewski, J. J., Cabrera, P. J., Saper, N. I. & Sanford, M. S. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 531, 220–224 (2016)
Zhang, F.-L., Hong, K., Li, T.-J., Park, H. & Yu, J.-Q. Functionalization of C(sp3)–H bonds using a transient directing group. Science 351, 252–256 (2016)
Fujiwara, Y. et al. Practical and innate carbon–hydrogen functionalization of heterocycles. Nature 492, 95–99 (2012)
Lin, S., Song, C.-X., Cai, G.-X., Wang, W.-H. & Shi, Z.-J. Intra/intermolecular direct allylic alkylation via Pd(II)-catalyzed allylic C–H activation. J. Am. Chem. Soc. 130, 12901–12903 (2008)
Wolff, M. E. Cyclization of N-halogenated amines (The Hofmann–Loffler reaction). Chem. Rev. 63, 55–64 (1963)
Bordwell, F. G., Zhang, S., Zhang, X.-M. & Liu, W.-Z. Homolytic bond dissociation enthalpies of the acidic H–A bonds caused by proximate substituents in sets of methyl ketones, carboxylic esters, and carboxamides related to changes in ground state energies. J. Am. Chem. Soc. 117, 7092–7096 (1995)
Bordwell, F. G. & Ji, G.-Z. Effects of structural changes on acidities and homolytic bond dissociation energies of the H–N bonds in amidines, carboxamides, and thiocarboxamides. J. Am. Chem. Soc. 113, 8398–8401 (1991)
Mayer, J. M. Proton-coupled electron transfer: a reaction chemist’s view. Annu. Rev. Phys. Chem. 55, 363–390 (2004)
Weinberg, D. R. et al. Proton-coupled electron transfer. Chem. Rev. 112, 4016–4093 (2012)
Reece, S. Y. & Nocera, D. G. Proton-coupled electron transfer in biology: results from synergistic studies in natural and model systems. Annu. Rev. Biochem. 78, 673–699 (2009)
Hanss, D., Freys, J. C., Bernardinelli, G. & Wenger, O. S. Cyclometalated iridium(III) complexes as photosensitizers for long-range electron transfer: occurrence of a Coulomb barrier. Eur. J. Inorg. Chem. 2009, 4850–4859 (2009)
Bortolamei, N., Isse, A. A. & Gennaro, A. Estimation of standard reduction potentials of alkyl radicals involved in atom transfer radical polymerization. Electrochim. Acta 55, 8312–8318 (2010)
Ward, W. M., Farnum, B. H., Siegler, M. & Meyer, G. J. Chloride ion-pairing with Ru(II) polypyridyl compounds in dichloromethane. J. Phys. Chem. A 117, 8883–8894 (2013)
Warren, J. J., Tronic, T. A. & Mayer, J. M. Thermochemistry of proton-coupled electron transfer reagents and its implications. Chem. Rev. 110, 6961–7001 (2010)
Acknowledgements
We thank the NIH (R01 GM113105) for financial support. R.R.K. is a Sloan Foundation Research Fellow and Amgen Young Investigator Award recipient. We also thank M. Rauch for assistance with quantum yield measurements and D. G. Oblinsky for assistance in measuring excited-state lifetimes. We thank E. Alexanian and R. Quinn (UNC Chapel Hill) for discussions. We thank T. Rovis (Colorado State University) for sharing results before publication.
Author information
Authors and Affiliations
Contributions
R.R.K. and G.J.C. conceived of the research. G.J.C., Q.Z. and D.C.M. carried out experiments and analysed results with R.R.K. C.J.G. assisted with substrate synthesis. R.R.K. wrote the manuscript with input from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks A. Studer and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data and additional references (see Contents for details). (PDF 20882 kb)
Rights and permissions
About this article
Cite this article
Choi, G., Zhu, Q., Miller, D. et al. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature 539, 268–271 (2016). https://doi.org/10.1038/nature19811
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19811
This article is cited by
-
Enantioselective synthesis of β- and α-amino ketones through reversible alkane carbonylation
Nature Synthesis (2024)
-
Oxidative two-state photoreactivity of a manganese(IV) complex using near-infrared light
Nature Chemistry (2024)
-
Photoenzymatic enantioselective intermolecular radical hydroamination
Nature Catalysis (2023)
-
Transition metal-free visible light photoredox-catalyzed remote C(sp3)−H borylation enabled by 1,5-hydrogen atom transfer
Communications Chemistry (2023)
-
Multi-site programmable functionalization of alkenes via controllable alkene isomerization
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.