Abstract
The transition from fins to limbs was an important terrestrial adaptation, but how this crucial evolutionary shift arose developmentally is unknown. Current models focus on the distinct roles of the apical ectodermal ridge (AER) and the signalling molecules that it secretes during limb and fin outgrowth. In contrast to the limb AER, the AER of the fin rapidly transitions into the apical fold and in the process shuts off AER-derived signals that stimulate proliferation of the precursors of the appendicular skeleton1,2,3,4,5,6,7,8,9,10. The differing fates of the AER during fish and tetrapod development have led to the speculation that fin-fold formation was one of the evolutionary hurdles to the AER-dependent expansion of the fin mesenchyme required to generate the increased appendicular structure evident within limbs11. Consequently, a heterochronic shift in the AER-to-apical-fold transition has been postulated to be crucial for limb evolution11. The ability to test this model has been hampered by a lack of understanding of the mechanisms controlling apical fold induction11,12. Here we show that invasion by cells of a newly identified somite-derived lineage into the AER in zebrafish regulates apical fold induction. Ablation of these cells inhibits apical fold formation, prolongs AER activity and increases the amount of fin bud mesenchyme, suggesting that these cells could provide the timing mechanism proposed in Thorogood’s clock model of the fin-to-limb transition11. We further demonstrate that apical-fold-inducing cells are progressively lost during gnathostome evolution; the absence of such cells within the tetrapod limb suggests that their loss may have been a necessary prelude to the attainment of limb-like structures in Devonian sarcopterygian fish.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Grandel, H., Draper, B. W. & Schulte-Merker, S. dackel acts in the ectoderm of the zebrafish pectoral fin bud to maintain AER signaling. Development 127, 4169–4178 (2000)
Géraudie, J. Initiation of the actinotrichial development in the early fin bud of the fish, Salmo. J. Morphol. 151, 353–361 (1977)
Saunders, J. W. Jr The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J. Exp. Zool 108, 363–403 (1948)
Todt, W. L. & Fallon, J. F. Development of the apical ectodermal ridge in the chick wing bud. J. Embryol. Exp. Morphol. 80, 21–41 (1984)
Fallon, J. F. & Kelley, R. O. Ultrastructural analysis of the apical ectodermal ridge during vertebrate limb morphogenesis. Development 51, 241–256 (1977)
Wood, A. Early pectoral fin development and morphogenesis of the apical ectodermal ridge in the killifish, Aphyosemion scheeli. Anat. Rec. 204, 349–356 (1982)
Dane, P. J. & Tucker, J. B. Modulation of epidermal cell shaping and extracellular matrix during caudal fin morphogenesis in the zebra fish Brachydanio rerio. J. Embryol. Exp. Morphol. 87, 145–161 (1985)
Jurand, A. Ultrastructural aspects of early development of the fore-limb buds in the chick and the mouse. Proc. R. Soc. Lond. B Biol. Sci. 162, 387–405 (1965)
Grandel, H. & Schulte-Merker, S. The development of the paired fins in the zebrafish (Danio rerio). Mech. Dev. 79, 99–120 (1998)
Yano, T., Abe, G., Yokoyama, H., Kawakami, K. & Tamura, K. Mechanism of pectoral fin outgrowth in zebrafish development. Development 139, 2916–2925 (2012)
Thorogood, P. in Developmental Patterning of the Vertebrate Limb (eds Hinchliffe, J. R. et al.) 347–354 (Springer US, 1991)
Yano, T. & Tamura, K. The making of differences between fins and limbs. J. Anat. 222, 100–113 (2013)
Neyt, C. et al. Evolutionary origins of vertebrate appendicular muscle. Nature 408, 82–86 (2000)
Christ, B., Jacob, H. J. & Jacob, M. Experimental analysis of the origin of the wing musculature in avian embryos. Anat. Embryol. (Berl.) 150, 171–186 (1977)
Chevallier, A., Kieny, M. & Mauger, A. Limb-somite relationship: origin of the limb musculature. J. Embryol. Exp. Morphol. 41, 245–258 (1977)
Mayeuf-Louchart, A. et al. Notch regulation of myogenic versus endothelial fates of cells that migrate from the somite to the limb. Proc. Natl Acad. Sci. USA 111, 8844–8849 (2014)
Goulding, M. D., Chalepakis, G., Deutsch, U., Erselius, J. R. & Gruss, P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 10, 1135–1147 (1991)
Gurevich, D. B. et al. Asymmetric division of clonal muscle stem cells coordinates muscle regeneration in vivo. Science http://dx.doi.org/10.1126/science.aad9969 (2016)
Kimmel, R. A. et al. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation Genes Dev. 14, 1377–1389 (2000)
Altabef, M., Clarke, J. D. & Tickle, C. Dorso-ventral ectodermal compartments and origin of apical ectodermal ridge in developing chick limb. Development 124, 4547–4556 (1997)
Zhang, J. et al. Loss of fish actinotrichia proteins and the fin-to-limb transition. Nature 466, 234–237 (2010)
Durán, I., Marí-Beffa, M., Santamaría, J. A., Becerra, J. & Santos-Ruiz, L. Actinotrichia collagens and their role in fin formation. Dev. Biol. 354, 160–172 (2011)
Webb, A. E. et al. Laminin α5 is essential for the formation of the zebrafish fins. Dev. Biol. 311, 369–382 (2007)
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl Acad. Sci. USA 99, 12651–12656 (2002)
Fischer, S., Draper, B. W. & Neumann, C. J. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development 130, 3515–3524 (2003)
Cohn, M. J., Izpisúa-Belmonte, J. C., Abud, H., Heath, J. K. & Tickle, C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80, 739–746 (1995)
Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) 4th edn (Univ. Oregon Press, 2000)
Ballard, W. W., Mellinger, J. & Lechenault, H. A series of normal stages for development of scyliorhinus-canicula, the lesser spotted dogfish (Chondrichthyes, scyliorhinidae). J. Exp. Zool. 267, 318–336 (1993)
Cole, N. J. et al. Development and evolution of the muscles of the pelvic fin. PLoS Biol. 9, e1001168 (2011)
Higashijima, S., Okamoto, H., Ueno, N., Hotta, Y. & Eguchi, G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev. Biol. 192, 289–299 (1997)
Proulx, K., Lu, A. & Sumanas, S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev. Biol. 348, 34–46 (2010)
Pisharath, H., Rhee, J. M., Swanson, M. A., Leach, S. D. & Parsons, M. J. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech. Dev. 124, 218–229 (2007)
Seger, C. et al. Analysis of Pax7 expressing myogenic cells in zebrafish muscle development, injury, and models of disease. Dev. Dyn. 240, 2440–2451 (2011)
Lee, R. T. H., Knapik, E. W., Thiery, J. P. & Carney, T. J. An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme. Development 140, 2923–2932 (2013)
Hatta, K., Tsujii, H. & Omura, T. Cell tracking using a photoconvertible fluorescent protein. Nat. Protocols 1, 960–967 (2006)
Nguyen, P. D. et al. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature 512, 314–318 (2014)
Pan, Y. A. et al. Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development 140, 2835–2846 (2013)
Pauls, S., Geldmacher-Voss, B. & Campos-Ortega, J. A. A zebrafish histone variant H2A.F/Z and a transgenic H2A.F/Z:GFP fusion protein for in vivo studies of embryonic development. Dev. Genes Evol. 211, 603–610 (2001)
Habuchi, S., Tsutsui, H., Kochaniak, A. B., Miyawaki, A. & van Oijen, A. M. mKikGR, a monomeric photoswitchable fluorescent protein. PLoS One 3, e3944–e3949 (2008)
Fisher, S. et al. Evaluating the biological relevance of putative enhancers using Tol2 transposon-mediated transgenesis in zebrafish. Nat. Protocols 1, 1297–1305 (2006)
Berger, J. et al. Loss of Tropomodulin4 in the zebrafish mutant trage causes cytoplasmic rod formation and muscle weakness reminiscent of nemaline myopathy. Dis. Model Mech. 7, 1407–1415 (2014)
Nguyen-Chi, M. E. et al. Morphogenesis and cell fate determination within the adaxial cell equivalence group of the zebrafish myotome. PLoS Genet. 8, e1003014–e1003016 (2012)
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protocols 3, 59–69 (2008)
Acknowledgements
The authors acknowledge E. McGlinn for expert advice, C. Wicking for comments on the manuscript, E. Tanaka for advice and technical support, Fishcore for expert zebrafish care, M. Morsch, V. Oorschot and the Monash electron microscopy facility for expert technical advice, Monash Micro Imaging for microscope availability and maintenance, S. Chow for technical assistance, M. Voz for reagents, T. Carney for fish lines and J. Joss for lungfish rearing and expertise. P.D.C. is supported by an NHMRC Principal Research Fellowship. N.J.C. is supported by The Snow Foundation and BitFury.org. This work was supported by an ARC Discovery Grant DP110101482. The Australian Regenerative Medicine Institute is supported by funds from the State Government of Victoria and the Australian Federal Government.
Reviewer Information Nature thanks C. Tabin and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
W.M. designed and performed experiments, analysed data and co-wrote the manuscript, N.J.C. designed and performed experiments, P.D.N., T.E.H., F.K. and G.W. generated reagents and fish strains, S.B., F.F., C.S., A.W. and N.C. performed experiments, P.D.C. designed and performed experiments, analysed data and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
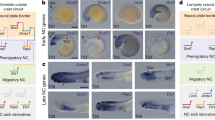
Extended Data Figure 1 Somitic mesoderm-derived cells migrate into the AER before AF formation and occupy a position distal to the AER basement membrane.
a–h, Somite-derived cells are positioned distally to the basement membrane of the AER. Pectoral fin of Tg(pax3a:GFP) transgenic embryos at 36 hpf (a–d, lateral view) and 48 hpf (e–h, transverse view), stained for pan-laminin (red), which marks the basement membrane of the AER (a, e), GFP (green) (b, f), DAPI (blue) (c, g), and a merged view (d, h). Arrowheads mark the somite-derived cells that invade the AER, and occupy a position distal to the basement membrane. i–l, Somite-derived cells are positioned within the AER and adopt expression of an epithelial marker. Dorsal view of a pectoral fin of a Tg(pax3a:GFP) embryo at 48 hpf, stained with an antibody against We3 (red), which is an epithelial marker for the AER and wound epithelium in salamander limbs (i), GFP (j), a merged view (k), and a merged differential contrast (DIC) image (l). Somite-derived cells (arrows) not only reside in the epithelial AER, but also co-express the epithelial marker. m–p, Histological analysis of the positioning of somite-derived cells within the AER and AF. Haematoxylin and eosin (H&E) staining (m, o) and periodic acid-Schiff (PAS) staining (n, p) on sagittal sections of Tg(pax3a:GFP) pectoral fins at 44 hpf (m, n) and 48 hpf (o, p). PAS specifically stains the basement membranes of tissues owing to the presence of Schiff-reactive glycoproteins within these structures. Arrows mark somitic mesoderm-derived cells in the AER (m, n) and AF (o, p), respectively. Both these histological analyses reveal that somite-derived cells are positioned distally to a continuous basement membrane. Scale bars, 20 μm.
Extended Data Figure 2 Distally located GFP-positive cells in the AER of Tg(pax3a:GFP) transgenic larvae do not contribute to either vasculature or musculature.
a–c, Tg(pax3a:GFP) does not label vasculature in the zebrafish pectoral fin. Medio-lateral view of Tg(pax3a:GFP); Tg(kdr1:mCherry) pectoral fins at 44 hpf (a), 48 hpf (b), and 52 hpf (c). Elongated GFP-positive cells localize distally to the mCherry-positive cells of the developing vasculature (d–g). Tg(pax3a:GFP)-expressing cells in the AER do not colocalize with Tg(actc1b:mCherry) expression (d). Dorsal and ventral muscle masses are defined by mCherry expression (e). GFP and mCherry colocalization shown in white (f). Merged image (g). Arrows mark GFP-positive cells in the AER, while arrowheads mark the vasculature. Scale bars, 50 μm.
Extended Data Figure 3 Validation of somite specific photoconversion of Tg(tbx6:gal4-vp16); Tg(UAS:Kaede) double-transgenic embryos.
a–q, Photoconversion of somite 4–7 (a–k) and somite 4 (l–q). a, Schematic representation of photoconversion. b–d, Dorsal views after photoconversion of somite 4–7 at the 15 somite stage reveals non-photoconverted Kaede (green) (b) and photoconverted Kaede (red) (c). Merged image shown in d. Somites 1–7 are numbered. e–g, Lateral views of the same photoconverted embryo at 2 dpf, revealing non-photoconverted Kaede (e) and a photoconverted Kaede (f). Merged image shown in g. h, Close-up view of photoconverted somites from e–g including brightfield. i–k, Close-up view of pectoral fin shown in e–g showing non-photoconverted Kaede (i) and photoconverted Kaede (j). Merged image shown in k. Asterisks indicate presumptive fin muscle progenitors, arrows mark photoconverted Kaede-positive cells of the PHM (e–g) and presumptive vasculature (i–k), arrowheads indicate photoconverted Kaede-positive cells in the AER. l–n, Dorsal view after photoconversion of somite 4 at 15 somites, showing non-photoconverted Kaede (l), photoconverted Kaede (m), and merged image (n). Somites 1–6 are numbered. o–q, Lateral view of the same photoconverted embryo at 2 dpf, showing non-photoconverted Kaede (o), photoconverted Kaede specifically in somite 4 (p), and a merged image (q). Somites 2–6 are numbered. Scale bars, 100 μm (b–g, i–q), 50 μm (h–k).
Extended Data Figure 4 Validation of homotopic somite transplantations.
a–d, Dorsal view of a somite 4 transplantation within a 13-somite stage embryo that has a Tg(ubi:zebrabow) (red) somite homotopically transplanted into a host embryo transgenic for Tg(actc1b:GFP), which marks the myotome in green. e–h, Dorsal view of the embryo in a–d at 24 hpf. i–l, Lateral view of the embryo in a–d at 2 dpf. m, Table of somite transplantations. Results from homotopic transplants of somites 3–6. n–p, Dorsal view of Tg(actc1b:GFP); Tg(pax3a:GFP) after somite 4 has been removed at the 15-somite stage. Solid lines indicate somite borders, numbers indicate somite identity. q–r, Lateral view of Tg(actc1b:GFP); Tg(pax3a:GFP) at 36 hpf. GFP-positive cells are present in the AER of both the control (q) and the experimental side (r) of the same embryo (n = 5). Scale bars, 100 μm (a–i), 50 μm (n–p) and 20 μm (q, r).
Extended Data Figure 5 Optimization of MTZ-induced ablations.
a–d, Tg(tbx6:gal4-vp16); Tg(UAS-E1b:Eco.NfsB-mCherry) embryos were treated with 0, 20 and 40 mM MTZ from 30–48 hpf and analysed at 48 hpf. a, Initial analyses assessed muscle transgene expression patterns, embryonic survival and overall phenotype severity as well as the efficiency of ablation of mCherry-expressing cells within the AF. b–d, Antibody stain for mCherry at 48 hpf reveals disrupted morphology of mesoderm-derived cells in the AF. Medio-lateral view of pectoral fins at 48 hpf treated with 0 mM MTZ (n = 8) (b), 20 mM MTZ (n = 7) (c), and 40 mM MTZ (n = 11) (d). Arrows indicate dead or dying cells based on cell morphology and fragmentation, dashed lines indicate the border between the AF and underlying mesenchyme. Scale bars, 50 μm.
Extended Data Figure 6 Optimization of the laser-ablation strategy.
a–l, Assessment of fluorescent recovery after laser ablation of Kaede-positive cells in the AER of double-transgenic Tg(tbx6:gal4-vp16); Tg(UAS:Kaede) embryos by scanning the region of interest (ROI) for 20 (a–c), 50 (d–f), 100 (g–i) and 200 (j–l) laser pulse iterations. m, Fluorescent intensity of the ROI before (scaled to 1, the initial fluorescence of the targeted cells) and after laser-mediated cell ablation. A very small amount of fluorescent recovery can be observed at 20 (c, m), 50 (f, m) and 100 (i, m) scan iterations, while 200 scan iterations results in a consistent and permanent loss of Kaede fluorescence (l, m). Scale bars, 10 μm.
Extended Data Figure 7 Validation of the laser-ablation strategy.
a–h, Morphological disruption after laser ablation of Kaede-positive cells in the AER of Tg(tbx6:gal4-vp16); Tg(UAS:Kaede) double-transgenic embryos. Arrows mark the ablated cell while the adjacent cell, indicated by arrowheads, is not affected. i–l, Membrane blebbing, as visualized by photoconverted Kaede (red), can be observed in the AER of Tg(tbx6:gal4-vp16); Tg(UAS:Kaede) double-transgenic embryos. Before ablation the cell is clearly labelled by non-photoconverted Kaede (green) (i), whereas after ablation, blebbing is observed at selected time points (arrows) (j–l). m–v, Proof-of-concept experiment in which each Kaede-positive cell in the AER is sequentially ablated from Tg(tbx6:gal4-vp16); Tg(UAS:Kaede) double-transgenic embryos. Arrows mark ablated cells. Ablation is cell-specific, and does not occur in non-targeted Kaede-positive cells in the AER or the underlying mesenchyme. w–zc, Random ablation of AER cells before AFIC invasion does not affect fin formation. Using Tg(h2a f/z:nlsKikGR1) transgenic embryos, in which all nuclei are labelled by the photoconvertible protein Kikume, it is possible to ablate cells at 32 hpf within the AER before AFIC invasion. Arrows indicate targeted cells within the AER at 2 s before ablation (w) and after ablation at 2 s (x), 12 s (y) and 22 s (z). za–zc, In each experimental condition, 6 cells were ablated per AER. No differences in actinotrichia formation or overall morphology can be detected when comparing contralateral control (za) with laser-ablated (zb) conditions. Detailed quantification of fin fold and mesenchyme length does not reveal any significant differences between contralateral control and laser-ablated conditions (zc). P values are by unpaired Student’s t-test. Scale bars, 10 μm (a–l), 50 μm (m–v), 10 μm (w–z) and 100 μm (za–zc). Error bars in zc represent s.e.m.
Extended Data Figure 8 Ablation of somite-derived cells from the AER results in increased proliferation of the underlying mesenchyme.
a–d, Ablation of somite-derived cells in the AER in embryos transgenic for Tg(tbx6:gal4-vp16) and Tg(UAS:Kaede) results in an increased mitotic index of the underlying mesenchyme as measured by EdU pulse labelling. All samples were analysed at 48 hpf. The endoskeletal disk is undergoing condensation in controls (arrowheads) (a, b), while this is not evident in MTZ-treated animals (arrow) (c). d, Quantification of EdU staining observed in the mesenchyme of pectoral fins after MTZ-induced ablation of somite-derived cells from the AER. e, Inhibition of Fgf signalling reduced the mitotic index in pectoral fins that have undergone MTZ-mediated ablation of somite-derived cells in the AER. The mitotic index of control fins is significantly lower compared to MTZ-treated pectoral fins. However, after treatment with the Fgf inhibitor SU5402, both MTZ-treated and untreated pectoral fins are reduced to a similar mitotic index. Scale bars, 40 μm. P values are by ANOVA with Tukey’s post-hoc analysis. Error bars in d and e represent s.e.m.
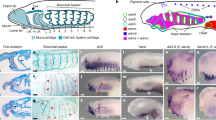
Extended Data Figure 9 Progressive reduction of collagen secreting cells from the AER of different species in the gnathostome phylogeny.
a–h, Embryos of the epaulette shark H. ocellatum stained for Col1a1a (red), skeletal muscle (green) and nuclei (blue). a, b, At 36 mm (approximately S. canicula stage 26/27; ref. 32), Col1a1-expressing cells are present at low numbers within the AER initially, with cells evident traversing the AER basement membrane from the fin mesenchyme (arrows in b, b’). The density of Col1a1a-expressing cells within the AER increases notably with the extension of the fin, and the cells are present in large numbers in 48 mm embryos (c, d), (approximately S. canicula stage 30/31; ref. 28). Col1a1a-expressing cells are maintained at the edge of the fin fold at advanced stages of fin development (85 mm, e, f) and cluster into discrete foci at the edge of the fin fold by 90 mm (g, h). a, c, e, g, Dorsal views proximal to the top, inserts are high-magnification views of the boxed regions showing only Col1a1a expression in red in each fin. b, d, f, h, Maximum projection confocal renders of the distal edge of the AER/AF region of each fin in a, c, e and g, respectively. b’, d’, f’, h’, Sagittal views of the rendered images in b, d, f and h, respectively, revealing the persistent localization of Col1a1a-expressing cells at the distal most edge of each fin (arrows). i–p, Embryos of the paddlefish P. spathula, sectioned in the transverse plane and stained for Col1a1a expression (brown, DAB) at 1 dpf, (i, j), 2 dpf (k, l) and 4 dpf (m, n). Colonization of the AER by Col1a1a-expressing cells follows a very similar pattern temporally and spatially to that evident in the zebrafish fin, but appear at a lower density than in the epaulette shark. o, p, Control immunohistochemistry on 1-dpf sections with no primary antibody. j, l, n, p, High-magnification views of the boxed regions in i, k, m and o, respectively. Arrows in j, l and n mark Col1a1a-expressing cells in the AER. q–x, N. forsteri embryos in transverse section and stained for Col1a1a and counterstained with H&E. Very few Col1a1a-expressing cells colonize the AER initially (q, r, stage 42–44), and they continue to be present in low numbers up to stage 48 (s, t) and are no longer evident at fin extension and AF formation stages (u, v, stage 50). w, x, Control immunohistochemistry on stage-48 sections with no primary antibody. r, t, v, x, High-magnification views of the boxed regions in q, s, u and w, respectively. Arrows in inset images in r and t mark the limited number of Col1a1a-expressing cells in the AER of lungfish embryos. Scale bars, 200 μm (a, c), 50 μm (b, f), 100 μm (d, h), 400 μm (e) and 500 μm (g, i, k, m, o, q, s, u, w).
Extended Data Figure 10 Schematic representation of pectoral fin development and its relation to the fin-to-limb transition.
a, Induction of fin fold formation and subsequent actinotrichia formation are dependent on somitic mesoderm-derived apical fold inducing cells (AFICs) cells infiltrating the AER. After the induction of fin bud formation (1), a somite-derived lineage (green) will infiltrate the AER (yellow, 2). Subsequent fin fold (red) induction (3) is dependent on this process, as is the formation of actinotrichia (dashed lines, 3 and 4), which rely on the secretion of collagen directly from these cells (green, 3 and 4). b, Throughout evolution of the gnathostome lineage, the cells that regulate fin fold induction have been gradually lost as a necessary prerequisite for limb formation. Schematic representation of fin development including the transition from AER (yellow) to AF (red). The timing of somite-derived cells (green bars) infiltrating the AER coincides with the AER to AF transition. Relative numbers of somite-derived cells are represented by the height of the green bars. In cartilaginous fish, cells infiltrate the AER early and abundantly to remain in the fin fold throughout development. In ray-finned fish, infiltration into the AER is delayed and the number of cells infiltrating the AER is also reduced, but infiltration continues throughout development. The number of cells infiltrating the AER and their timing of residency is further reduced in lobe-finned fish, while finally in tetrapods there is an absence of somite-derived cells in the AER, and a lack of AF induction.
Supplementary information
Time-lapse recording of cell migration to the fin bud from the somites in TgBAC(pax3a:GFP) transgenic embryos
Time-lapse recording corresponding to images presented in Fig. 1a-c. Analysis of animals transgenic for TgBAC(pax3a:GFP) from 23 hpf – 33 hpf identifies that somite 4-6 contributes to the zebrafish pectoral fin mesenchyme (pfm) while somite 7 contributes to the posterior hypaxial muscle (phm) (n=6). Anterior is to the left, dorsal to the top, view is lateral. (MOV 6055 kb)
Time-lapse recording of pectoral fin bud development in Tg(pax3a:GFP) transgenic embryos
Time-lapse recording corresponding to images presented in Fig. 1d-i. GFP positive cells originating in the pectoral fin bud mesenchyme migrate outside of the cluster of GFP positive cells in the fin bud mesenchyme and take on an elongated morphology within the AER. Anterior is to the left dorsal to the top, view is lateral. (MOV 2982 kb)
Maximum projection and surface rendering of the homotopic transplantation of somite 4
Maximum projection followed by a surface rendering of the images presented in Fig. 2i-r. In this analysis somite 4 from an embryo transgenic for Tg(ubi:zebrabow) (red) is homotopically transplanted into a host embryo transgenic for Tg(actc1b:GFP) which marks the myotome in green. Contribution of cells derived from the grafted somite (red) to the AER is observed. Embryo is 48hpf, anterior is to the left dorsal to the top, initial view is lateral. (MOV 19424 kb)
Laser mediated ablation of Tg(tbx6:gal4-vp16); Tg(UAS:Kaede) positive cells in the AER results in cell blebbing
Video recording of images presented in Extended Data Fig. 7i-l. A 405nm diode laser was used to ablate a region of interest specific for a cell in the AER containing non-photoconverted Kaede (green). After ablation non-photoconverted Kaede is absent and photoconverted Kaede (red) remains. Overall cellular morphology is disrupted and blebbing of dying cells is observed immediately post-irradiation. Anterior is to the left dorsal to the top, view is lateral. (MOV 10819 kb)
Rights and permissions
About this article
Cite this article
Masselink, W., Cole, N., Fenyes, F. et al. A somitic contribution to the apical ectodermal ridge is essential for fin formation. Nature 535, 542–546 (2016). https://doi.org/10.1038/nature18953
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18953
This article is cited by
-
Hoxd13/Bmp2-mediated mechanism involved in zebrafish finfold design
Scientific Reports (2021)
-
ECM alterations in Fndc3a (Fibronectin Domain Containing Protein 3A) deficient zebrafish cause temporal fin development and regeneration defects
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.