Abstract
Neurons can release damaged mitochondria and transfer them to astrocytes for disposal and recycling1. This ability to exchange mitochondria may represent a potential mode of cell-to-cell signalling in the central nervous system. Here we show that astrocytes in mice can also release functional mitochondria that enter neurons. Astrocytic release of extracellular mitochondrial particles was mediated by a calcium-dependent mechanism involving CD38 and cyclic ADP ribose signalling. Transient focal cerebral ischaemia in mice induced entry of astrocytic mitochondria into adjacent neurons, and this entry amplified cell survival signals. Suppression of CD38 signalling by short interfering RNA reduced extracellular mitochondria transfer and worsened neurological outcomes. These findings suggest a new mitochondrial mechanism of neuroglial crosstalk that may contribute to endogenous neuroprotective and neurorecovery mechanisms after stroke.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
14 September 2016
A Correction to this paper has been published: https://doi.org/10.1038/nature19805
References
Davis, C. H. et al. Transcellular degradation of axonal mitochondria. Proc. Natl Acad. Sci. USA 111, 9633–9638 (2014)
Iadecola, C. & Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376 (2007)
Attwell, D. et al. Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010)
Khakh, B. S. & Sofroniew, M. V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952 (2015)
Rosenberg, P. A. & Aizenman, E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci. Lett. 103, 162–168 (1989)
Wang, X. F. & Cynader, M. S. Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J. Neurosci. 21, 3322–3331 (2001)
Ouyang, Y. B. et al. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia 61, 1784–1794 (2013)
Nagai, M. et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 10, 615–622 (2007)
Haidet-Phillips, A. M. et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat. Biotechnol. 29, 824–828 (2011)
Voloboueva, L. A., Suh, S. W., Swanson, R. A. & Giffard, R. G. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J. Neurochem. 102, 1383–1394 (2007)
Anne Stetler, R., Leak, R. K., Gao, Y. & Chen, J. The dynamics of the mitochondrial organelle as a potential therapeutic target. J. Cereb. Blood Flow Metab. 33, 22–32 (2013)
Falchi, A. M. et al. Astrocytes shed large membrane vesicles that contain mitochondria, lipid droplets and ATP. Histochem. Cell Biol. 139, 221–231 (2013)
Islam, M. N. et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 18, 759–765 (2012)
Aksoy, P., White, T. A., Thompson, M. & Chini, E. N. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun. 345, 1386–1392 (2006)
Guse, A. H. & Lee, H. C. NAADP: a universal Ca2+ trigger. Sci. Signal. 1, re10 (2008)
Bruzzone, S. et al. Glutamate-mediated overexpression of CD38 in astrocytes cultured with neurones. J. Neurochem. 89, 264–272 (2004)
Levy, A. et al. CD38 facilitates recovery from traumatic brain injury. J. Neurotrauma 26, 1521–1533 (2009)
Higashida, H. et al. Social memory, amnesia, and autism: brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem. Int. 61, 828–838 (2012)
Choe, C. U. et al. CD38 exacerbates focal cytokine production, postischemic inflammation and brain injury after focal cerebral ischemia. PLoS One 6, e19046 (2011)
Frühbeis, C. et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 11, e1001604 (2013)
Bowen, S. et al. The phagocytic capacity of neurones. Eur. J. Neurosci. 25, 2947–2955 (2007)
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011)
Hu, X. et al. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 11, 56–64 (2015)
Xin, H. et al. Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PLoS One 5, e9027 (2010)
Hayakawa, K., Pham, L. D., Katusic, Z. S., Arai, K. & Lo, E. H. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc. Natl Acad. Sci. USA 109, 7505–7510 (2012)
Proia, P. et al. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int. J. Mol. Med. 21, 63–67 (2008)
Li, Y., Liu, Z., Xin, H. & Chopp, M. The role of astrocytes in mediating exogenous cell-based restorative therapy for stroke. Glia 62, 1–16 (2014)
Lo, E. H. Degeneration and repair in central nervous system disease. Nat. Med. 16, 1205–1209 (2010)
Xing, C. & Lo, E. H. Help-me signaling: Non-cell autonomous mechanisms of neuroprotection and neurorecovery. Prog. Neurobiol. http://dx.doi.org/10.1016/j.pneurobio.2016.04.004 (2016)
Rah, S. Y., Park, K. H., Han, M. K., Im, M. J. & Kim, U. H. Activation of CD38 by interleukin-8 signaling regulates intracellular Ca2+ level and motility of lymphokine-activated killer cells. J. Biol. Chem. 280, 2888–2895 (2005)
Graeff, R. M., Walseth, T. F., Fryxell, K., Branton, W. D. & Lee, H. C. Enzymatic synthesis and characterizations of cyclic GDP-ribose. A procedure for distinguishing enzymes with ADP-ribosyl cyclase activity. J. Biol. Chem. 269, 30260–30267 (1994)
Bi, B. et al. Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury. J. Neurosci. 31, 9205–9221 (2011)
Cruz, F. C. et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat. Rev. Neurosci. 14, 743–754 (2013)
Vogel, R. et al. Quantitative sizing of nano/microparticles with a tunable elastomeric pore sensor. Anal. Chem. 83, 3499–3506 (2011)
Hayakawa, K. et al. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 30, 871–882 (2010)
Hayakawa, K., Arai, K. & Lo, E. H. Role of ERK map kinase and CRM1 in IL-1β-stimulated release of HMGB1 from cortical astrocytes. Glia 58, 1007–1015 (2010)
Dehmelt, L., Poplawski, G., Hwang, E. & Halpain, S. NeuriteQuant: an open source toolkit for high content screens of neuronal morphogenesis. BMC Neurosci. 12, 100 (2011)
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (NIH), the Rappaport Foundation, and the China National Natural Science Foundation Award For Distinguished Young Scholars. Electron microscopy was performed in the Center for Systems Biology. Cytometric assessments were supported by the Department of Pathology Flow and Image Cytometry Core. The authors thank J. Felton and J. Zwicker for assistance with qNano analysis.
Author information
Authors and Affiliations
Contributions
K.H. contributed to manuscript preparation, hypothesis generation, experimental design/analysis and conducted experiments. E.E., X.W., Y.T., Y.L. and C.X. conducted experiments and helped with data analysis. X.J. and E.H.L. contributed to manuscript preparation, hypothesis generation and experimental design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Astrocytic mitochondria particle detection.
a, Electron microscopic analysis demonstrated that mitochondria were detected within extracellular astrocyte-derived particles. Free mitochondria were also found in ACM. b, In FACS analysis, control beads were used to gate populations ranging in size from 500 to 900 nm. c, In ACM, approximately 53% of particles in the size range were positive for functional mitochondria (n = 2 biological replicates, n = 5 independent experiments). d, After FACS analysis to isolate the extracellular mitochondria fraction from ACM, particle size was measured by qNano analysis. Consistent with electron microscopy analysis, a range of size distributions was observed (~25%: 300–400 nm, ~75%: 400–1,100 nm). Data are mean ± s.e.m.
Extended Data Figure 2 Characteristics of astrocytic mitochondrial particles in FACS analysis.
a, Mitochondrial particles were identified by FACS. b, Of these mitochondrial particles, FACS analysis showed that approximately 79% and 43% of particles express β1-integrin and CD63, respectively (n = 2 biological replicates, n = 4 independent experiments). cADPR (1 μM) did not appear to affect these distributions (n = 2 biological replicates, n = 4 independent experiments). Data are mean ± s.e.m.
Extended Data Figure 3 Production of astrocytic mitochondrial particles in a Ca2+-dependent mechanism.
a, The known CD38 downstream signal, cADPR, increased intracellular calcium levels shown by Fluo-4 intensity in a concentration-dependent manner (n = 3 independent experiments). b, Intracellular ATP levels in astrocytes were upregulated by cADPR stimulation (n = 4 independent experiments). **P < 0.01 versus 0 μM cADPR. c, To measure ATP levels in extracellular particles, ACM was collected and large debris was excluded by centrifugation and filtration through a 1.2-μm filter. After another centrifugation at 20,000g for 30 min, 100 μl from the top or bottom fractions were used for the ATP assay. d, The bottom fraction had a higher ATP content, and cADPR (1 μM) increased the ATP content in this fraction (n = 2 biological replicates, n = 8 or 5 independent experiments). e, cADPR-induced extracellular ATP levels within extracellular particles were diminished by the intracellular calcium blocker, BAPTA-AM (n = 2 biological replicates, n = 6 or 4 independent experiments). Data are mean ± s.e.m. P values are from a one-way ANOVA followed by Tukey’s test.
Extended Data Figure 4 Summary of experiment in
Fig. 2c . a, We repeated the experiment in Fig. 2c with n = 4 independent primary cultures per group. Similar results were obtained (n = 2 biological replicates, n = 4 independent experiments). The extracellular mdACM group was significantly different compared to the ACM group. Furthermore, in this repeated experiment, there was also statistical significance between controls (OGD-damaged neurons alone) versus those treated with mitochondria-containing astrocyte media (ACM), and there was no statistically significant worsening when comparing control versus mitochondria-depleted groups (mdACM). Taken together, these two separate experiments suggest a modest but statistically significant neuroprotection induced by astrocyte-derived mitochondria. Data are mean ± s.e.m. one-way ANOVA followed by Tukey’s test. b, MitoTracker Red CMXRos (200 nM) was incubated without astrocytes to obtain no-cell-derived media (negative control). Media was collected and further incubated with neurons after OGD. After 24 h, there was no mitochondrial signal observed. Scale bars, 100 μm.
Extended Data Figure 5 Role of astrocytic CD38 in mitochondria transfer during starvation in vitro.
a, Immunocytochemistry in neuron–astrocyte co-cultures demonstrated that CD38 was primarily expressed within astrocytes. b, Extracellular ATP levels were higher in media collected from neurons co-cultured with astrocytes compared to media from neuron-alone cultures (n = 3 biological replicates, n = 9 or 11 independent experiments). c, After serum/glucose starvation, neurons were significantly damaged, as expected. But neurons co-cultured with astrocytes were protected (n = 2 biological replicates, n = 6 or 4 independent experiments). d, CD38 suppression by siRNA significantly decreased extracellular ATP levels in neuron–astrocyte co-cultures, but CD38 suppression did not affect extracellular ATP levels in neuron-alone cultures (n = 3 biological replicates, n = 9 or 6 independent experiments). e, Blockade of astrocytic CD38 by siRNA significantly increased LDH release (indicative of cell damage) in the co-cultures, suggesting that CD38 may be important for maintaining neuroglial homeostasis (n = 2 biological replicates, n = 6 independent experiments). f, Rat primary neurons were co-cultured with rat astrocytes. Immunocytochemistry showed that CD38 suppression by siRNA reduced astrocytic mitochondria (red) transfer into neurons compared to control. g, h, Western blot analysis indicated that CD38 suppression by siRNA can be successfully performed in astrocyte culture without affecting cell viability (n = 2 biological replicates, n = 4 or 3 independent experiments). Data are mean ± s.e.m. P values are from an unpaired t-test.
Extended Data Figure 6 Metabolic inhibition in astrocyte causes neuronal cell death and prevents neurite outgrowth in vitro.
a, Astrocytic aconitase was inhibited by fluorocitrate (FC), which disrupted astrocyte metabolism that was accompanied by a senescence-associated β-galactosidase (SA-β-gal) signal. b, Intracellular ATP was decreased in these metabolically disrupted astrocytes (n = 2 biological replicates, n = 6 independent experiments). *P < 0.05, **P < 0.01 versus 0 mM fluorocitrate. c, Propidium iodide (PI) staining showed that fluorocitrate (0.5 mM) did not induce cell death in astrocytes. d, Metabolically disrupted astrocytes significantly decreased the mitochondrial membrane potential. Red: aggregated JC1; green: monomer JC1. Scale bar, 20 μm. e, Rat cortical neurons were co-cultured with JC1-labelled astrocytes. After 24 h co-culture, control astrocytes transferred mitochondria, which had a high-membrane potential (aggregated JC1), but metabolically disrupted astrocytes released and transferred dysfunctional mitochondria into neurons (n = 3 independent experiments). f, Metabolically disrupted astrocytes could not support neural viability under starvation in the co-culture (n = 4 independent experiments). g, Co-culture between astrocytes and neurons was conducted for 48 h to test neurite outgrowth. Immunocytochemistry showed that metabolically disrupted astrocytes prevented neurite outgrowth and increased neuronal cell death (n = 3 independent experiments). h, The LDH assay indicated that fluorocitrate (0.5 mM) did not affect cell viability in either rat cortical astrocytes (n = 4 independent experiments) or rat cortical neurons (n = 4 independent experiments). Data are mean ± s.e.m. P values are from a one-way ANOVA followed by Tukey’s test (b) or an un unpaired t-test (e–g).
Extended Data Figure 7 FACS analysis using E17 FVB/N-Tg (GFAPGFP)14Mes/J transgenic mice.
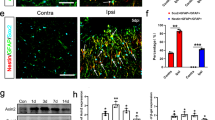
a, Cortical neurons were isolated from E17 FVB/N-Tg (GFAPGFP)14Mes/J transgenic mice. Immunocytochemistry showed that cultured neurons did not express either GFP or GFAP protein after OGD, suggesting that stroke-like stress may not lead to ‘leakiness’ in this astrocyte-specific GFP mouse. b, Brain cell suspension was prepared from FVB/N-Tg (GFAPGFP)14Mes/J mice subjected to transient ischaemia, then FACS analysis was performed. c, Representative image before cell sorting. d, Purity after cell sorting. e, The MAP2+ GFP− or MAP2+ GFP+ populations were 92.5% or 85.9% positive for DAPI, respectively. f, Western blot analysis demonstrated that both GFP-positive and -negative neurons expressed mature neuron marker (neurofilament) but not neuronal stem-cell marker (nestin). These data exclude the possibility that GFAP-positive cells included subsets of neuronal precursor cells that are known to also express GFAP.
Extended Data Figure 8 Effects of CD38 suppression by siRNA in vivo and in vitro.
a, Western blot showed that CD38 expression was increased in peri-infarct cortex at days 1 to 7 after stroke. b, Cd38 siRNA or a scrambled control was injected into lateral ventricles at 5 days after stroke. Western blot analysis confirmed that CD38 expression was successfully decreased in peri-infarct cortex at 7 days. c, In peri-infarct cortex, CD8 T-cell and Iba1-positive microglia/macrophages were detected by immunohistochemistry. d, Quantification of the number of CD8-positive or Iba1-positive cells indicated that there was no difference between control siRNA or Cd38 siRNA (n = 6 mice per group). Data are mean ± s.e.m. e, Cultured rat cortical astrocytes were subjected to OGD for 2 h followed by treating with control siRNA or Cd38 siRNA. Astrocyte cell morphology or GFAP expression was assessed by immunocytochemistry or western blot after 22 h reoxygenation. f, Morphology change was not clearly observed in cultured astrocytes treated with Cd38 siRNA compared to control siRNA. g, Western blot analysis showed that CD38 was successfully decreased by siRNA transfection but GFAP expression was not changed.
Extended Data Figure 9 Neuronal purity confirmed by FACS analysis in vivo.
To confirm our FACS findings, we used two different published standard approaches32,33. a, By FACS, the MAP2-positive population was gated and further assessed by markers such as Iba1 (microglia/macrophage) and GFAP (astrocyte) in brain cell samples isolated from C57BL/6 J mice. These comparisons confirmed that the MAP2+ population did not contain any appreciable amounts of microglia or astrocytes, whereas another neuron marker (NeuN) was highly enriched. b, Similar findings were obtained using an alternative gating method to isolate neurons.
Extended Data Figure 10 Involvement of integrin-mediated Src/Syk signalling mechanisms in astrocytic mitochondrial entry into neurons in vitro.
a, b, Cultured rat cortical astrocytes were stimulated by cADPR (1 μM) for 24 h. Intracellular mitochondria labelled by MitoTracker dye was significantly increased in astrocytes stimulated with cADPR (1 μM) (n = 2 biological replicates, n = 7 independent experiments). **P < 0.01 versus 0 h. c, Some mitochondria were found outside of cells. d, FACS analysis revealed that approximately 5 × 105 mitochondria were contained in 1 ml ACM. cADPR (1 μM) significantly increased the number of mitochondria in the media (n = 2 biological replicates, n = 6 independent experiments). e, Experimental schedule to quantify the entry of mitochondria into neurons after OGD. Rat cortical neurons (1 × 105 cells per well) were prepared in 24-well culture plates. ACM or cADPR-ACM (each 1 ml) was co-incubated with neurons for 18 h. Mitochondrial entry into neurons was calculated by mitochondrial intensity measured before and after washing cells with PBS. Phenol-red-free culture media was used to decrease the background signal. The background signal was subtracted from the fluorescent intensity obtained from each sample. f, OGD for 2 h decreased approximately 50% of the mitochondria in neurons after 18 h reoxygenation (n = 2 biological replicates, n = 4 independent experiments). g, Data are expressed as relative values, with total neuronal mitochondria after 2 h OGD/18 h reoxygenation being 100%. Mitochondrial entry into neurons was slightly higher after cADPR-ACM treatment (18%) compared to ACM treatment (11%), although there was no statistical significance (n = 2 biological replicates, n = 4 independent experiments). h, There was no difference in the percentage of mitochondrial entry after ACM treatment or cADPR-ACM treatment (n = 2 biological replicates, n = 4 independent experiments). i, cADPR-ACM treatment supported neuronal viability better than ACM treatment (n = 2 biological replicates, n = 4 independent experiments). j, Co-culture between rat cortical astrocytes in the upper chamber and rat cortical neurons in the lower chamber was performed for 18 h after OGD for 2 h in neurons. Mitochondrial entry into neurons was then measured. k, Immediately after OGD, dynasore (5 μM), RGDS peptide (H-Arg-Gly-Asp-Ser-OH; 50 μg ml−1), or MNS (3,4-methylenedioxy-β-nitrostyrene; 1 μM) was initially added to neurons for 30 min, then astrocyte co-culture was performed for 18 h. Data are expressed as relative values, with astrocytic extracellular mitochondria plus entered mitochondria into neurons being 100%. RGDS peptide and MNS significantly decreased mitochondrial entry into neurons, but dynasore did not inhibit the entry. l, MNS treatment significantly decreased astrocyte-mediated neuroprotection (n = 2 biological replicates, n = 4 independent experiments). m, Dynasore (5 μM), RGDS peptide (50 μg ml−1), or MNS (1 μM) did not affect neuronal viability after 2 h OGD (n = 4). Data are mean ± s.e.m. These data suggest that the entry of astrocytic mitochondrial particles into neurons may involve integrin-mediated Src/Syk signalling mechanisms. However, we acknowledge that these pathways may be multifactorial, and deeper analyses are warranted to dissect entry mechanisms under various physiological and pathological conditions. P values are from a one-way ANOVA followed by Tukey’s test (a, i, k, l) or an unpaired t-test (d, g).
Rights and permissions
About this article
Cite this article
Hayakawa, K., Esposito, E., Wang, X. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016). https://doi.org/10.1038/nature18928
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18928
This article is cited by
-
ROS/mtROS promotes TNTs formation via the PI3K/AKT/mTOR pathway to protect against mitochondrial damages in glial cells induced by engineered nanomaterials
Particle and Fibre Toxicology (2024)
-
Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice
Journal of Neuroinflammation (2024)
-
A repair pathway lost in multiple sclerosis provides a new drug opportunity
Nature Immunology (2024)
-
Exploring the limitations of mitochondrial dye as a genuine horizontal mitochondrial transfer surrogate
Communications Biology (2024)
-
Mitochondria as secretory organelles and therapeutic cargos
Experimental & Molecular Medicine (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.