Abstract
Maladaptive aggressive behaviour is associated with a number of neuropsychiatric disorders1 and is thought to result partly from the inappropriate activation of brain reward systems in response to aggressive or violent social stimuli2. Nuclei within the ventromedial hypothalamus3,4,5, extended amygdala6 and limbic7 circuits are known to encode initiation of aggression; however, little is known about the neural mechanisms that directly modulate the motivational component of aggressive behaviour8. Here we established a mouse model to measure the valence of aggressive inter-male social interaction with a smaller subordinate intruder as reinforcement for the development of conditioned place preference (CPP). Aggressors develop a CPP, whereas non-aggressors develop a conditioned place aversion to the intruder-paired context. Furthermore, we identify a functional GABAergic projection from the basal forebrain (BF) to the lateral habenula (lHb) that bi-directionally controls the valence of aggressive interactions. Circuit-specific silencing of GABAergic BF–lHb terminals of aggressors with halorhodopsin (NpHR3.0) increases lHb neuronal firing and abolishes CPP to the intruder-paired context. Activation of GABAergic BF–lHb terminals of non-aggressors with channelrhodopsin (ChR2) decreases lHb neuronal firing and promotes CPP to the intruder-paired context. Finally, we show that altering inhibitory transmission at BF–lHb terminals does not control the initiation of aggressive behaviour. These results demonstrate that the BF–lHb circuit has a critical role in regulating the valence of inter-male aggressive behaviour and provide novel mechanistic insight into the neural circuits modulating aggression reward processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Anderson, D. J. Optogenetics, sex, and violence in the brain: implications for psychiatry. Biol. Psychiatry 71, 1081–1089 (2012)
Decety, J., Michalska, K. J., Akitsuki, Y. & Lahey, B. B. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol. Psychol. 80, 203–211 (2009)
Yang, C. F. et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909 (2013)
Wasman, M. & Flynn, J. P. Directed attack elicited from hypothalamus. Arch. Neurol. 6, 220–227 (1962)
Lin, D. et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011)
Unger, E. K. et al. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Reports 10, 453–462 (2015)
Yu, Q. et al. Optogenetic stimulation of DAergic VTA neurons increases aggression. Mol. Psychiatry 19, 635 (2014)
Takahashi, A. & Miczek, K. A. Neurogenetics of aggressive behavior: studies in rodents. Curr. Top. Behav. Neurosci. 17, 3–44 (2013)
Kudryavtseva, N. N., Bakshtanovskaya, I. V. & Koryakina, L. A. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 38, 315–320 (1991)
Golden, S. A., Covington, H. E. III, Berton, O. & Russo, S. J. A standardized protocol for repeated social defeat stress in mice. Nat. Protocols 6, 1183–1191 (2011)
Miczek, K. A., DeBold, J. F. & Thompson, M. L. Pharmacological, hormonal, and behavioral manipulations in analysis of aggressive behavior. Prog. Clin. Biol. Res. 167, 1–26 (1984)
Glenn, A. L. & Yang, Y. The potential role of the striatum in antisocial behavior and psychopathy. Biol. Psychiatry 72, 817–822 (2012)
Zahm, D. S., Parsley, K. P., Schwartz, Z. M. & Cheng, A. Y. On lateral septum-like characteristics of outputs from the accumbal hedonic “hotspot” of Peciña and Berridge with commentary on the transitional nature of basal forebrain “boundaries”. J. Comp. Neurol. 521, 50–68 (2013)
Callaway, E. M. & Luo, L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J. Neurosci. 35, 8979–8985 (2015)
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012)
Herkenham, M. & Nauta, W. J. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J. Comp. Neurol. 173, 123–145 (1977)
Sutherland, R. J. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 6, 1–13 (1982)
Lecca, S., Meye, F. J. & Mameli, M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur. J. Neurosci. 39, 1170–1178 (2014)
Shelton, L. et al. Mapping pain activation and connectivity of the human habenula. J. Neurophysiol. 107, 2633–2648 (2012)
Hikosaka, O. The habenula: from stress evasion to value-based decision-making. Nature Rev. Neurosci. 11, 503–513 (2010)
Lee, E. H. & Huang, S. L. Role of lateral habenula in the regulation of exploratory behavior and its relationship to stress in rats. Behav. Brain Res. 30, 265–271 (1988)
Maroteaux, M. & Mameli, M. Cocaine evokes projection-specific synaptic plasticity of lateral habenula neurons. J. Neurosci. 32, 12641–12646 (2012)
Li, B. et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470, 535–539 (2011)
Yetnikoff, L., Cheng, A. Y., Lavezzi, H. N., Parsley, K. P. & Zahm, D. S. Sources of input to the rostromedial tegmental nucleus, ventral tegmental area, and lateral habenula compared: a study in rat. J. Comp. Neurol. 523, 2426–2456 (2015)
Quina, L. A. et al. Efferent pathways of the mouse lateral habenula. J. Comp. Neurol. 523, 32–60 (2015)
Felton, T. M., Linton, L., Rosenblatt, J. S. & Morell, J. I. First and second order maternal behavior related afferents of the lateral habenula. Neuroreport 10, 883–887 (1999)
Beck, A., Heinz, A. J. & Heinz, A. Translational clinical neuroscience perspectives on the cognitive and neurobiological mechanisms underlying alcohol-related aggression. Curr. Top. Behav. Neurosci. 17, 443–474 (2014)
Martin, L. A., Neighbors, H. W. & Griffith, D. M. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA Psychiatry 70, 1100–1106 (2013)
Bewernick, B. H., Kayser, S., Sturm, V. & Schlaepfer, T. E. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology 37, 1975–1985 (2012)
Sartorius, A. & Henn, F. A. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med. Hypotheses 69, 1305–1308 (2007)
Kudryavtseva, N. N., Bondar, N. P. & Avgustinovich, D. F. Association between experience of aggression and anxiety in male mice. Behav. Brain Res. 133, 83–93 (2002)
Russo, S. J. et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J. Neurosci. 29, 3529–3537 (2009)
Golden, S. A. et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nature Med. 19, 337–344 (2013)
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007)
Yang, M., Silverman, J. L. & Crawley, J. N. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. Chapter 8, Unit 8 26 (2011)
Golde, W. T., Gollobin, P. & Rodriguez, L. L. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim. (NY) 34, 39–43 (2005)
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013)
Friedman, A. K. et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344, 313–319 (2014)
Lobo, M. K. et al. ΔFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J. Neurosci. 33, 18381–18395 (2013)
Lobo, M. K. et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390 (2010)
Stamatakis, A. M. & Stuber, G. D. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nature Neurosci. 15, 1105–1107 (2012)
Chandra, R. et al. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front. Mol. Neurosci. 6, 13 (2013)
Aquili, L., Liu, A. W., Shindou, M., Shindou, T. & Wickens, J. R. Behavioral flexibility is increased by optogenetic inhibition of neurons in the nucleus accumbens shell during specific time segments. Learn. Mem. 21, 223–231 (2014)
Kadir, S. N., Goodman, D. F. M. & Harris, K. D. High-dimensional cluster analysis with the masked EM algorithm. Neural Comput. 26, 2379–2394 (2014)
Acknowledgements
This research was supported by US National Institutes of Health grants R01 MH090264, P50 MH096890 and P50 AT008661-01 (S.J.R.), R01 MH092306 (M.H.H.), T32 MH087004 (M.L.P., M.H. and M.F.), T32 MH096678 (M.L.P.), F30 MH100835 (M.H.), F31 MH105217 (M.L.P.), National Institute of General Medical Sciences 1FI2GM117583-01 (S.A.G.) and the Natural National Science Foundation of China 81200862 (H.Z.). We would like to thank K. Miczek and Y. Shaham for their input.
Author information
Authors and Affiliations
Contributions
S.A.G. and S.J.R. designed and wrote the manuscript. S.A.G., D.J.C., M.H., C.M., J.J.W., M.L.P., N.R. H.A., G.E.H., M.F., D.B., L.K., J.T. and B.K. collected behavioural and immunohistochemistry data and aided in data analysis. H.Z., M.-H.H., D.C., K.G. and M.L.S. designed, carried out and analysed electrophysiological experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks O. Hikosaka and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Social behaviours exhibited by resident CD-1 and intruder C57 mice during aggression screening.
a, Experimental schematic of aggression screening procedure used in a subset (40 residents and 40 intruders) of mice to quantify social behaviours. b–e, Bouts of attacks (F2,156 = 13.10, two-way ANOVA ***P < 0.0001; post-hoc test ***P < 0.001; n = 40 per group) (b), pursuits (c), withdrawals (F2,156 = 5.745, two-way repeated measures ANOVA **P < 0.001; post-hoc test ***P < 0.001; n = 40 per group) (d) and non-aggressive social approaches (e). f–h, Duration of attacks (F2,156 = 7.069, two-way repeated measures ANOVA **P < 0.001; post-hoc test ***P < 0.001; n = 40 per group) (f), pursuits (g), withdrawals (h) and non-aggressive social approaches (e). All data are presented as mean ± s.e.m.
Extended Data Figure 2 Detailed ethological analysis of AGG aggression-related behaviours.
a, Experimental schematic of aggression screening procedure used in a sample (448 mice total; 138 NON and 310 AGG) of mice. b, Histogram of attack latency frequency using 10-s bins. c–e, Mean distribution across screening sessions (left) and individual screening sessions (right) for latency to aggression (F2,1338 = 49.37, two-way repeated measures ANOVA P < 0.001; post-hoc test, *P < 0.001; n = 138–310) (c), number of attack bouts (F2,1338 = 21.03, two-way repeated measures ANOVA P < 0.001; post-hoc test, *P < 0.001; n = 138–310) (d) and mean attack duration (F2,1338 = 11.96, two-way repeated measures ANOVA P < 0.001; post-hoc test, *P < 0.001; n = 138–310) (e). f, g, Correlation of mean latency to initial aggression with mean attack bouts (r = −0.78, P < 0.0001) (f) and mean duration of attack bouts (r = −0.40, P < 0.0001) (g). Distribution plots are presented as the median with interquartile range and normality determined by D’Agostino–Pearson, Shapiro–Wilk and Kolmogorov–Smirnov normality tests (P < 0.0001). Summary data are represented as mean ± s.e.m.
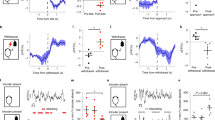
Extended Data Figure 3 Aggression CPP behaviour.
a, Experimental schematic of aggression CPP procedure. b, c, Individual duration spent in the intruder-paired context for AGG (t7 = 3.106, *P < 0.05; two-tailed paired t-test, n = 8 per group) (b) and NON (t7 = 2.918, *P < 0.05; two-tailed paired t-test, n = 8 per group) (c). d, Duration spent in the middle neutral chamber during pre-test and test sessions. e, Experimental schematic of sensory CPP procedure. f, g, Individual duration spent in the intruder-paired context for AGG (f) and NON (g). h, Duration spent in the middle neutral chamber during pre-test and test sessions. Summary data are represented as mean ± s.e.m.
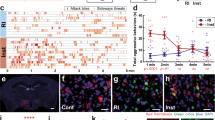
Extended Data Figure 4 BF–lHb circuit tracing and GABAergic cell-type specificity.
a, Schematic of viral tracing strategy. b, Representative BF viral infection with AAV2-hSyn-eYFP. Scale bar: 500 μm. c, Histological analysis of viral infection with AAV2-hSyn-eYFP (F3,11 = 223.0, one-way ANOVA ***P < 0.0001, post-hoc test, ***P < 0.0001; n = 3 mice, 3 slices per mouse) across adjacent anatomical regions. d, e, Whole-cell electrophysiological recordings (d) and representative traces of lHb neurons photostimulated with AAV2-hSyn-ChR2.0 in the absence or presence of bath-applied GABAA receptor antagonist gabazine (2 μm; F2,7 = 220, one-way ANOVA P < 0.05; post-hoc test, ***P < 0.001, n = 4, 2, 2 cells from 2 mice) (e). f, Optically evoked IPSC response delay (n = 21 oIPSC events, 2 mice). g, Representative images of eYFPBF→lHb terminal colocalization between vesicular GABA transporter (top), and not vesicular glutamate transporter 1 (bottom). Scale bars: 10 μm; white arrows indicate colocalization within insets. MS, medial septum; pLS, posterior lateral septum. Summary data are represented as mean ± s.e.m.
Extended Data Figure 5 Multiunit anaesthetized optrode recordings.
a, Schematic of in vivo anaesthetized multi-unit optrode recording procedure (left) and representative optrode placement in lHb (right; scale bar: 200 μm). b, c, Heatmaps of normalized firing rates for lHb neurons in response to BF terminal stimulation with ChR2BF→lHb (b) or NpHR3BF→lHb (c) and averaged spike wave-form shown below for pre-stimulation, stimulation and post-stimulation epochs. d, Percentage of cells by firing response (top) and average normalized lHb firing rate (bottom) after BF–lHb terminal stimulation with ChR2BF→lHb for all identified cells (F2,134 = 8.249, one-way repeated-measure ANOVA P < 0.001; post-hoc test, *P < 0.05; n = 68 cells from 3 mice) and cells that significantly decreased firing during the stimulation epoch (F7,105 = 8.868, one-way repeated-measure ANOVA P < 0.0001; post-hoc test, *P < 0.05; n = 16/68 cells from 3 mice). e, Percentage of cells by firing response (top) and average normalized lHb firing rate (bottom) after BF–lHb terminal stimulation with NpHR3BF→lHb for all identified cells (F2,128 = 10.32, one-way repeated-measure ANOVA P < 0.0001; post-hoc test, *P < 0.05; n = 65/65 cells from 3 mice) and cells that significantly increased firing during the stimulation epoch (F7,203 = 17.58, one-way repeated-measure ANOVA P < 0.0001; post-hoc test, *P < 0.05; n = 30/65 cells from 3 mice). mHb, medial habenula. Summary data are represented as mean ± s.e.m.
Extended Data Figure 6 BF–lHb AAV infection and CPP locomotor behaviour.
a, Schematic of BF coronal slice (left), alongside representative AAV-ChR2-eYFP (top) and AAV-NpHR3.0-eYFP (bottom) infections. Scale bar: 500 μm. b, Schematic of lHb coronal slice (left), alongside representative images of BF terminal infection by AAV-ChR2-eYFP (middle top) and AAV-NpHR3.0-eYFP (middle bottom) within the lHb. Scale bar: 200 μm. Representative close-ups of terminal regions shown in insets on right. Scale bar: 50 μm. All representative images counterstained with DAPI. c, d, Histological analysis of BF infection in NON (c) and AGG (d) mice. e, f, Histological analysis of habenular viral infection in NON (e) and AGG mice (f). g–j, Total distance travelled (g, h) and mean velocity (i, j) between NON and AGG during the CPP pre-test and test phase. All data are presented as mean ± s.e.m., and are not significant as determined by two-way ANOVA, P < 0.05. dStr, dorsal striatum; mHb, medial habenula; MS, medial septum; pLS, posterior lateral septum.
Extended Data Figure 7 Direct lHb stimulation bi-directionally modulates aggression reward.
a, Schematic of viral infection strategy. b, c, Representative images of lHb cell body infection in NON (b) and AGG (c). Scale bar: 200 μm. d, Histological analysis of lHb viral infection. e, Representative CPP traces of NON. NON::NpHRlHb cell body infection mimics the physiological effect of NON::ChR2BF→lHb terminal stimulation. f, Normalized CPP score (t15 = 2.834, *P < 0.05; two-tailed unpaired t-test, n = 8–9 per group) and subtracted CPP score (t15 = 3.058, **P < 0.01; two-tailed unpaired t-test, n = 8–9 per group) in NON::eYFPlHb and NON::NpHRlHb. g, Individual duration spent in the intruder-paired context for NON::eYFPlHb (t9 = 0.9129, P > 0.05; two-tailed paired t-test, n = 10 per group) and NON::NpHRlHb (t9 = 2.344, *P < 0.05; two-tailed paired t-test, n = 10 per group). h, Representative CPP traces of AGG::eYFPlHb and AGG::ChR2lHb. i, Normalized CPP score (t18 = 2.692, *P < 0.05; two-tailed unpaired t-test, n = 9–11 per group) and subtracted CPP score (t18 = 4.203, ***P < 0.01; two-tailed unpaired t-test, n = 9–11 per group) for the intruder-paired context in AGG::eYFPlHb and AGG::ChR2lHb. j, Individual duration spent in the intruder-paired context for AGG::eYFPlHb mice (t10 = 3.212, **P < 0.01; two-tailed paired t-test, n = 9 per group) and AGG::ChR2lHb mice (t8 = 1.348, P < 0.05; two-tailed paired t-test, n = 11 per group). Summary data are represented as mean ± s.e.m. dStr, dorsal striatum; mHb, medial habenula.
Extended Data Figure 8 BF–lHb stimulation modulates cocaine CPP.
a, Experimental timeline of general anxiety and cocaine CPP testing. b–e, BF–lHb stimulation during open field testing (b, c) and elevated plus maze testing (d, e). f, Subthreshold cocaine (10 mg kg−1, intraperitoneal) CPP procedure with BF–lHb stimulation during CPP test (t9 = 2.403, P < 0.05; two-tailed unpaired t-test, n = 5–6 per group).
Rights and permissions
About this article
Cite this article
Golden, S., Heshmati, M., Flanigan, M. et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692 (2016). https://doi.org/10.1038/nature18601
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18601
This article is cited by
-
The risk of being bitten by a dog is higher on hot, sunny, and smoggy days
Scientific Reports (2023)
-
An appetite for aggressive behavior? Female rats, too, derive reward from winning aggressive interactions
Translational Psychiatry (2023)
-
Effects of early life stress and subsequent re-exposure to stress on neuronal activity in the lateral habenula
Neuropsychopharmacology (2023)
-
Urocortin-3 neurons in the perifornical area are critical mediators of chronic stress on female infant-directed behavior
Molecular Psychiatry (2023)
-
Median raphe serotonergic neurons projecting to the interpeduncular nucleus control preference and aversion
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.