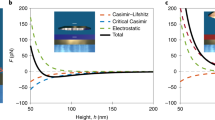
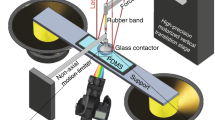
Abstract
When a gecko moves on a ceiling it makes use of adhesion and stiction. Stiction—static friction—is experienced on microscopic and macroscopic scales and is related to adhesion and sliding friction1. Although important for most locomotive processes, the concepts of adhesion, stiction and sliding friction are often only empirically correlated. A more detailed understanding of these concepts will, for example, help to improve the design of increasingly smaller devices such as micro- and nanoelectromechanical switches2. Here we show how stiction and adhesion are related for a liquid drop on a hexagonal boron nitride monolayer on rhodium3, by measuring dynamic contact angles in two distinct states of the solid–liquid interface: a corrugated state in the absence of hydrogen intercalation and an intercalation-induced flat state. Stiction and adhesion can be reversibly switched by applying different electrochemical potentials to the sample, causing atomic hydrogen to be intercalated or not. We ascribe the change in adhesion to a change in lateral electric field of in-plane two-nanometre dipole rings4, because it cannot be explained by the change in surface roughness known from the Wenzel model5. Although the change in adhesion can be calculated for the system we study6, it is not yet possible to determine the stiction at such a solid–liquid interface using ab initio methods. The inorganic hybrid of hexagonal boron nitride and rhodium is very stable and represents a new class of switchable surfaces with the potential for application in the study of adhesion, friction and lubrication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Persson, B. N. J. Sliding Friction (Springer, 1998)
Wagner, T. J. W. & Vella, D. Switch on, switch off: stiction in nanoelectromechanical switches. Nanotechnology 24, 275501 (2013)
Corso, M. et al. Boron nitride nanomesh. Science 303, 217–220 (2004)
Dil, H. et al. Surface trapping of atoms and molecules with dipole rings. Science 319, 1824–1826 (2008)
Wenzel, R. N. Surface roughness and contact angle. J. Phys. Chem. 53, 1466–1467 (1949)
Golze, D., Hutter, J. & Iannuzzi, M. Wetting of water on hexagonal boron nitride@Rh(111): a QM/MM model based on atomic charges derived for nanostructured substrates. Phys. Chem. Chem. Phys. 17, 14307–14316 (2015)
Ternes, M., Lutz, C. P., Hirjibehedin, C. F., Giessibl, F. J. & Heinrich, A. J. The force needed to move an atom on a surface. Science 319, 1066–1069 (2008)
Krim, J., Solina, D. H. & Chiarello, R. Nanotribology of a Kr monolayer: a quartz crystal microbalance study of atomic-scale friction. Phys. Rev. Lett. 66, 181–184 (1991)
Ehrlich, G. & Hudda, F. Atomic view of surface self-diffusion: tungsten on tungsten. J. Chem. Phys. 44, 1039–1049 (1966)
Evans, M. G. & Polanyi, M. Inertia and driving force of chemical reactions. Trans. Faraday Soc. 34, 11–24 (1938)
Zhang, Q., Qi, Y., Hector, L. G. Jr, Cagin, T. & Goddard, W. A. III. Origin of static friction and its relationship to adhesion at the atomic scale. Phys. Rev. B 75, 144114 (2007)
Ichimura, K., Oh, S.-K. & Nakagawa, M. Light-driven motion of liquids on a photoresponsive surface. Science 288, 1624–1626 (2000)
Sun, T. et al. Reversible switching between superhydrophilicity and superhydrophobicity. Angew. Chem. Int. Ed. 43, 357–360 (2004)
Lahann, J. et al. A reversibly switching surface. Science 299, 371–374 (2003)
Berner, S. et al. Boron nitride nanomesh: functionality from a corrugated monolayer. Angew. Chem. Int. Ed. 46, 5115–5119 (2007)
Widmer, R. et al. Electrolytic in situ STM investigation of h-BN-nanomesh. Electrochem. Commun. 9, 2484–2488 (2007)
Brugger, T. et al. Nanotexture switching of single-layer hexagonal boron nitride on rhodium by intercalation of hydrogen atoms. Angew. Chem. Int. Ed. 49, 6120–6124 (2010)
Willman, K. W. & Murray, R. W. Contact angle between water and a poly(vinylferrocene) film on a potential-controlled platinum electrode. Anal. Chem. 55, 1139–1142 (1983)
Sung, Y. E., Thomas, S. & Wieckowski, A. Characterization of the Rh(111) electrode by CEELS, AES, LEED, and voltammetry. Adsorption of (bi)sulfate, perchlorate, and carbon monoxide. J. Phys. Chem. 99, 13513–13521 (1995)
Colonell, J. I., Curtiss, T. J. & Sibener, S. J. Coverage dependence of the kinetics for H2 desorption from Rh(111). Surf. Sci. 366, 19–28 (1996)
Hu, S. et al. Proton transport through one-atom-thick crystals. Nature 516, 227–230 (2014)
Marx, D., Tuckerman, M. E., Hutter, J. & Parrinello, M. The nature of the hydrated excess proton in water. Nature 397, 601–604 (1999)
de Gennes, P. G. Wetting: statics and dynamics. Rev. Mod. Phys. 57, 827–863 (1985)
Kwok, D. Y. & Neumann, A. W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 81, 167–249 (1999)
Amirfazli, A., Hanig, S., Müller, A. & Neumann, A. W. Measurements of line tension for solid–liquid–vapor systems using drop size dependence of contact angles and its correlation with solid–liquid interfacial tension. Langmuir 16, 2024–2031 (2000)
Schneemilch, M., Welters, W. J. J., Hayes, R. A. & Ralston, J. Electrically induced changes in dynamic wettability. Langmuir 16, 2924–2927 (2000)
Manukyan, G., Oh, J. M., van den Ende, D., Lammertink, R. G. H. & Mugele, F. Electrical switching of wetting states on superhydrophobic surfaces: a route towards reversible Cassie-to-Wenzel transitions. Phys. Rev. Lett. 106, 014501 (2011)
Schrader, M. E. Young–Dupré revisited. Langmuir 11, 3585–3589 (1995)
Iannuzzi, M. et al. Site-selective adsorption of phthalocyanine on h-BN/Rh(111) nanomesh. Phys. Chem. Chem. Phys. 16, 12374–12384 (2014)
Ma, H. et al. Chiral distortion of confined ice oligomers (n = 5,6). Langmuir 28, 15246–15250 (2012)
Ding, Y., Iannuzzi, M. & Hutter, J. Investigation of boron nitride nanomesh interacting with water. J. Phys. Chem. C 115, 13685–13692 (2011)
Holzschuh, E., Fritschi, M. & Kündig, W. Measurement of the electron neutrino mass from tritium β-decay. Phys. Lett. B 287, 381–388 (1992)
Hemmi, A. et al. High quality single atomic layer deposition of hexagonal boron nitride on single crystalline Rh(111) four-inch wafers. Rev. Sci. Instrum. 85, 035101 (2014)
King, S. W., Nemanich, R. J. & Davis, R. F. Cleaning of pyrolytic hexagonal boron nitride surfaces. Surf. Interface Anal. 47, 798–803 (2015)
Cun, H., Iannuzzi, M., Hemmi, A., Osterwalder, J. & Greber, T. Two-nanometer voids in single-layer hexagonal boron nitride: formation via the “can-opener” effect and annihilation by self-healing. ACS Nano 8, 7423–7431 (2014)
Hansen, W. N. & Kolb, D. M. The work function of emersed electrodes. J. Electroanal. Chem. 100, 493–500 (1979)
Koper, M. T. M. Blank voltammetry of hexagonal surfaces of Pt-group metal electrodes: comparison to density functional theory calculations and ultra-high vacuum experiments on water dissociation. Electrochim. Acta 56, 10645–10651 (2011)
Jerkiewicz, G. & Zolfaghari, A. Determination of the energy of the metal–underpotential-deposited hydrogen bond for rhodium electrodes. J. Phys. Chem. 100, 8454–8461 (1996)
Zolfaghari, A., Chayer, M. & Jerkiewicz, G. Energetics of the underpotential deposition of hydrogen on platinum electrodes. I. Absence of coadsorbed species. J. Electrochem. Soc. 144, 3034–3041 (1997)
Łosiewicz, B., Jurczakowski, R. & Lasia, A. Kinetics of hydrogen underpotential deposition at polycrystalline rhodium in acidic solutions. Electrochim. Acta 56, 5746–5753 (2011)
Acknowledgements
We acknowledge financial support by the Swiss National Science Foundation within the funding instrument ‘Sinergia’. S.F.L.M. acknowledges the receipt of an FP7 Marie Curie European reintegration grant, ERC grant OxideSurfaces and, together with S.D.F., support by FWO–Vlaanderen. We thank M. Schmid for help with the depiction of the STM images, G. Schütz for discussions and A. P. Seitsonen for the artwork in Fig. 1g.
Author information
Authors and Affiliations
Contributions
T.G. conceived the project together with S.F.L.M., who designed and performed the electrochemical experiments, in situ STM and in situ contact angle measurements. A.H. prepared the nanomesh samples, analysed contact angle data and performed the TDS measurements. S.M. built the contact angle apparatus. O.G. drew our attention to dynamic contact angle measurements. S.D.F. and J.O. managed the Sinergia project. All authors contributed to discussions. S.F.L.M., A.H. and T.G. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Thermal desorption spectroscopy.
a, h-BN/Rh(111) thin-film sample and sample holder ready for the desorption experiment in the ultrahigh-vacuum chamber. b, Thermal desorption experiment with temperature and power data shown in the top panel and pressure data in the bottom panel. The purple data are pyrometer readings, which were calibrated to a previous thermocouple measurement using a Rh crystal. The simulated temperature is a solution of equations (1) and (2) with the applied power and a starting temperature of 25 °C as input variables.
Extended Data Figure 2 Electrochemical STM during hydrogen intercalation.
The substrate potential during scanning (image scanned from bottom to top) was switched from E1 = 0 V (deintercalated, yellow arrow) to E2 = −0.25 V (intercalated, light blue arrow) at about one-fifth of the way from the lower edge of the STM image. On the timescale of imaging, intercalation-induced flattening of the surface is instantaneous, in sharp contrast to deintercalation (compare with Fig. 1e). Image size, 66 nm × 66 nm; tunnelling current, 0.1 nA; tip potential fixed at −0.45 V.
Supplementary information
Advancing, followed by receding electrolyte drop on h-BN/Rh(111), at an applied substrate potential E = +0.1 V
Advancing, followed by receding electrolyte drop on h-BN/Rh(111), at an applied substrate potential E = +0.1 V, where the nanomesh occurs in its normal corrugated state. The video shows all images captured between t = 68 s and t = 80 s in Figure 3a. (MOV 4472 kb)
Advancing, followed by receding electrolyte drop on h-BN/Rh(111), at an applied substrate potential E = –0.35 V
Advancing, followed by receding electrolyte drop on h-BN/Rh(111), at an applied substrate potential E = –0.35 V, where hydrogen intercalation has caused flattening of the nanomesh. The video shows all images captured between between t = 95 s and t = 115 s in Figure 3b. (MOV 7657 kb)
Rights and permissions
About this article
Cite this article
Mertens, S., Hemmi, A., Muff, S. et al. Switching stiction and adhesion of a liquid on a solid. Nature 534, 676–679 (2016). https://doi.org/10.1038/nature18275
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18275
This article is cited by
-
Solvent-free carbon sphere nanofluids towards intelligent lubrication regulation
Friction (2024)
-
Proton transport through nanoscale corrugations in two-dimensional crystals
Nature (2023)
-
Gate-controlled suppression of light-driven proton transport through graphene electrodes
Nature Communications (2023)
-
Wien effect in interfacial water dissociation through proton-permeable graphene electrodes
Nature Communications (2022)
-
Proton-assisted growth of ultra-flat graphene films
Nature (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.