Abstract
The distribution, accumulation and circulation of oxygen and hydrogen in Earth’s interior dictate the geochemical evolution of the hydrosphere, atmosphere and biosphere1. The oxygen-rich atmosphere and iron-rich core represent two end-members of the oxygen–iron (O–Fe) system, overlapping with the entire pressure–temperature–composition range of the planet. The extreme pressure and temperature conditions of the deep interior alter the oxidation states1, spin states2 and phase stabilities3,4 of iron oxides, creating new stoichiometries, such as Fe4O5 (ref. 5) and Fe5O6 (ref. 6). Such interactions between O and Fe dictate Earth’s formation, the separation of the core and mantle, and the evolution of the atmosphere. Iron, in its multiple oxidation states, controls the oxygen fugacity and oxygen budget, with hydrogen having a key role in the reaction of Fe and O (causing iron to rust in humid air). Here we use first-principles calculations and experiments to identify a highly stable, pyrite-structured iron oxide (FeO2) at 76 gigapascals and 1,800 kelvin that holds an excessive amount of oxygen. We show that the mineral goethite, FeOOH, which exists ubiquitously as ‘rust’ and is concentrated in bog iron ore, decomposes under the deep lower-mantle conditions to form FeO2 and release H2. The reaction could cause accumulation of the heavy FeO2-bearing patches in the deep lower mantle, upward migration of hydrogen, and separation of the oxygen and hydrogen cycles. This process provides an alternative interpretation for the origin of seismic and geochemical anomalies in the deep lower mantle, as well as a sporadic O2 source for the Great Oxidation Event over two billion years ago that created the present oxygen-rich atmosphere.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Frost, D. J. & McCammon, C. A. The redox state of Earth’s mantle. Annu. Rev. Earth Planet. Sci. 36, 389–420 (2008)
Badro, J. et al. Iron partitioning in Earth's mantle: toward a deep lower mantle discontinuity. Science 300, 789–791 (2003)
Ono, S. & Ohishi, Y. In situ X-ray observation of phase transformation in Fe2O3 at high pressures and high temperatures. J. Phys. Chem. Solids 66, 1714–1720 (2005)
Bykova, E. et al. Structural complexity of simple Fe2O3 at high pressures and temperatures. Nature Commun. 7, 10661 (2016)
Lavina, B. et al. Discovery of the recoverable high-pressure iron oxide Fe4O5 . Proc. Natl Acad. Sci. USA 108, 17281–17285 (2011)
Lavina, B. & Meng, Y. Unraveling the complexity of iron oxides at high pressure and temperature: synthesis of Fe5O6 . Sci. Adv. 1, e1400260 (2015)
Meng, Y., Hrubiak, R., Rod, E., Boehler, R. & Shen, G. New developments in laser-heated diamond anvil cell with in-situ x-ray diffraction at High Pressure Collaborative Access Team. Rev. Sci. Instrum. 86, 072201 (2015)
Akahama, Y. & Kawamura, H. High-pressure Raman spectroscopy of solid oxygen. Phys. Rev. B 54, R15602–R15605 (1996)
Sørensen, H. O. et al. Multigrain crystallography. Z. Kristallogr. 227, 63–78 (2012)
Zhang, L. et al. Single-crystal structure determination of (Mg,Fe)SiO3 postperovskite. Proc. Natl Acad. Sci. USA 110, 6292–6295 (2013)
Zhang, L. et al. Disproportionation of (Mg,Fe)SiO3 perovskite in Earth’s deep lower mantle. Science 344, 877–882 (2014)
Fujihisa, H. et al. O8 cluster structure of the epsilon phase of solid oxygen. Phys. Rev. Lett. 97, 085503 (2006)
Gleason, A. E., Jeanloz, R. & Kunz, M. Pressure–temperature stability studies of FeOOH using X-ray diffraction. Am. Mineral. 93, 1882–1885 (2008)
Howie, R. T., Dalladay-Simpson, P. & Gregoryanz, E. Raman spectroscopy of hot hydrogen above 200 GPa. Nature Mater. 14, 495–499 (2015)
Loubeyre, P., Letoullec, R. & Pinceaux, J. P. Properties of H2 under strong compression in a Ne matrix. Phys. Rev. Lett. 67, 3271–3274 (1991)
Jacobsen, S. D. & van der Lee, S. (eds) Earth's Deep Water Cycle Vol. 168 (American Geophysical Union, 2006)
Nishi, M. et al. Stability of hydrous silicate at high pressures and water transport to the deep lower mantle. Nature Geosci. 7, 224–227 (2014)
Pamato, M. G. et al. Lower-mantle water reservoir implied by the extreme stability of a hydrous aluminosilicate. Nature Geosci. 8, 75–79 (2014)
Schmandt, B., Jacobsen, S. D., Becker, T. W., Liu, Z. & Dueker, K. G. Dehydration melting at the top of the lower mantle. Science 344, 1265–1268 (2014)
Oganov, A. R., Lyakhov, A. O. & Valle, M. How evolutionary crystal structure prediction works—and why. Acc. Chem. Res. 44, 227–237 (2011)
Weerasinghe, G. L., Pickard, C. J. & Needs, R. J. Computational searches for iron oxides at high pressures. J. Phys. Condens. Matter 27, 455501 (2015)
Dziewonski, A. & Anderson, D. L. Preliminary reference earth model. Phys. Earth Planet. Inter. 25, 297–356 (1981)
Stagno, V., Ojwang, D. O., McCammon, C. A. & Frost, D. J. The oxidation state of the mantle and the extraction of carbon from Earth's interior. Nature 493, 84–88 (2013)
Li, M., McNamara, A. K. & Garnero, E. J. Chemical complexity of hotspots caused by cycling oceanic crust through mantle reservoirs. Nature Geosci. 7, 366–370 (2014)
Hirose, K., Fei, Y., Ma, Y. & Mao, H. K. The fate of subducted basaltic crust in the Earth's lower mantle. Nature 397, 53–56 (1999)
Ren, Z. Y., Ingle, S., Takahashi, E., Hirano, N. & Hirata, T. The chemical structure of the Hawaiian mantle plume. Nature 436, 837–840 (2005)
Hutko, A. R., Lay, T., Garnero, E. J. & Revenaugh, J. Seismic detection of folded, subducted lithosphere at the core–mantle boundary. Nature 441, 333–336 (2006)
Holzapfel, C., Rubie, D., Frost, C. & Langenhorst, F. Fe-Mg interdiffusion in (Mg,Fe)SiO3 perovskite and lower mantle reequilibration. Science 309, 1707–1710 (2005)
Yang, J. et al. Diamonds, native elements and metal alloys from chromitites of the Ray-Iz ophiolite of the Polar Urals. Gondwana Res. 27, 459–485 (2015)
Lyons, T. W., Reinhard, C. T. & Planavsky, N. J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506, 307–315 (2014)
Canfield, D. E. The early history of atmospheric oxygen: homage to Robert M. Garrels. Annu. Rev. Earth Planet. Sci. 33, 1–36 (2005)
Farquhar, J., Bao, H. & Thiemens, M. Atmospheric influence of Earth’s earliest sulfur cycle. Science 289, 756–758 (2000)
Shim, S.-H. & Duffy, T. S. Raman spectroscopy of Fe2O3 to 62 GPa. Am. Mineral. 87, 318–326 (2002)
Fei, Y. et al. Toward an internally consistent pressure scale. Proc. Natl Acad. Sci. USA 104, 9182–9186 (2007)
Loubeyre, P., Letoullec, R. & Pinceaux, J. P. Raman measurements of the vibrational properties of H2 as a guest molecule in dense helium, neon, argon, and deuterium systems up to 40 GPa. Phys. Rev. B 45, 12844–12853 (1992)
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964)
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965)
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994)
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992)
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996)
Baroni, S., de Gironcoli, S., Dal Corso, A. & Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 73, 515–562 (2001)
Togo, A. & Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 108, 1–5 (2015)
Acknowledgements
We thank Y. Fei for providing haematite powder samples; A. Goncharov for conducting the laser-heating treatment; and D. Z. Zhang, J.-F. Shu, J. Smith, Y. Kono, K. Yang, S. Yan, Z. H. Yu, Y. Yuan, M.Q. Hou and L. Xu for beamline technical support. XRD measurements were performed at the High Pressure Collaborative Access Team (HPCAT 16-IDB and 16-BMD) Advanced Photon Source (APS), Argonne National Laboratory, and the BL15U1 beamline, Shanghai Synchrotron Radiation Facility in China. Part of the experiments was performed at the 13BM-C experimental station of the GeoSoilEnviroCARS facility at the APS. HPCAT operations are supported by the DOE-NNSA under award number DE-NA0001974 and by the DOE-BES under award number DE-FG02-99ER45775, with partial instrumentation funding by the NSF. 13BM-C operation is supported by COMPRES through the Partnership for Extreme Crystallography (PX2) project, under NSF Cooperative Agreement EAR 11-57758. APS is supported by the DOE-BES, under contract number DE-AC02-06CH11357. Q.H. and H.-K.M. were supported by NSF grants EAR-1345112 and EAR-1447438. L.Z. was supported by the Foundation of President of China Academy of Engineering Physics (grant no. 201402032) and the National Natural Science Foundation of China (grant no. 41574080). This work was also supported in part by the National Natural Science Foundation of China (grant number U1530402).
Author information
Authors and Affiliations
Contributions
W.Y., L.Y. and Q.H. carried out the experiment. Y.M. conducted the laser heating for the first synthesis of FeO2. W.Y., L.Z., Q.H. and H.-K.M. performed the experimental data analysis. D.Y.K. predicted the FeO2 structure and performed the computer simulation. H.-K.M. conceived and designed the project and directed the calculations and experiments. Q.H. and H.-K.M. wrote the manuscript. All authors contributed to the discussion of the results and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Bonding lengths and angles in pyrite-type FeO2 at 76 GPa.
The structure is viewed along the x axis of the experimental FeO2 unit cell.
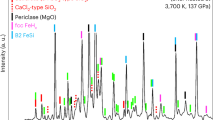
Extended Data Figure 2 XRD pattern series by decompressing the P-phase.
The P-phase becomes weak in intensity at 41 GPa and totally disappears at 31 GPa and below. The decompressed sample eventually recovered to the α-Fe2O3 phase at low pressure. P indicates the P-phase FeO2; O indicates solid O2; H indicates α-Fe2O3 (haematite). Fe2O3 contains post-perovskite type and Aba2-structured high-pressure phases.
Extended Data Figure 3 Synthesis pressure–temperature conditions for FeO2.
a, Open circles indicate the coexistence of Fe2O3 and O2. Solid squares indicate the appearance of P-phase FeO2. FeO2 was synthesized between 72 GPa and 75 GPa. b, Open circles indicate FeOOH. Decomposition pressure was constrained between 78 GPa and 87 GPa. Sample temperature was measured using the spectroradiometric method and errors are estimated from the goodness of fit to the spectroradiometric profile.
Extended Data Figure 4 XRD patterns of FeOOH through the experimental pressure–temperature path.
a, Goethite sample (G) in neon and Re gasket at 0.9 GPa. b, As in a, compressed to 35 GPa. The XRD peaks include goethite, solidified Ne, and the Re gasket. c, As in a, compressed to 92 GPa. The sample peaks remain and shift to higher Q. d, After laser-heating and quenching to ambient temperature, the pressure dropped to 87 GPa, and the goethite peaks disappeared. The new pattern consists of peaks of the P-phase, Ne and minor amounts of ε-FeOOH (red stars).
Extended Data Figure 5 Phonon dispersion relations of FeO2 P-phase.
a, At 0 GPa; b, At 300 GPa. The P-phase is mechanically stable at 0 GPa and 300 GPa.
Rights and permissions
About this article
Cite this article
Hu, Q., Kim, D., Yang, W. et al. FeO2 and FeOOH under deep lower-mantle conditions and Earth’s oxygen–hydrogen cycles. Nature 534, 241–244 (2016). https://doi.org/10.1038/nature18018
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18018
This article is cited by
-
Pressure-induced large volume collapse and possible spin transition in HP-PdF2-type FeCl2
Physics and Chemistry of Minerals (2024)
-
Plate Tectonics: The Stabilizer of Earth’s Habitability
Journal of Earth Science (2023)
-
Slab control on the mega-sized North Pacific ultra-low velocity zone
Nature Communications (2022)
-
Mineralogical Aspects of Reducing Lump Iron Ore, Pellets, and Sinter with Hydrogen
Metallurgical and Materials Transactions B (2022)
-
Notes for a History of Gas Geochemistry
Journal of Earth Science (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.