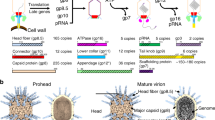
Abstract
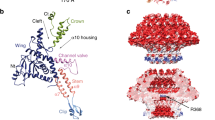
Most bacteriophages are tailed bacteriophages with an isometric or a prolate head attached to a long contractile, long non-contractile, or short non-contractile tail1. The tail is a complex machine that plays a central role in host cell recognition and attachment, cell wall and membrane penetration, and viral genome ejection. The mechanisms involved in the penetration of the inner host cell membrane by bacteriophage tails are not well understood. Here we describe structural and functional studies of the bacteriophage ϕ29 tail knob protein gene product 9 (gp9). The 2.0 Å crystal structure of gp9 shows that six gp9 molecules form a hexameric tube structure with six flexible hydrophobic loops blocking one end of the tube before DNA ejection. Sequence and structural analyses suggest that the loops in the tube could be membrane active. Further biochemical assays and electron microscopy structural analyses show that the six hydrophobic loops in the tube exit upon DNA ejection and form a channel that spans the lipid bilayer of the membrane and allows the release of the bacteriophage genomic DNA, suggesting that cell membrane penetration involves a pore-forming mechanism similar to that of certain non-enveloped eukaryotic viruses2,3,4. A search of other phage tail proteins identified similar hydrophobic loops, which indicates that a common mechanism might be used for membrane penetration by prokaryotic viruses. These findings suggest that although prokaryotic and eukaryotic viruses use apparently very different mechanisms for infection, they have evolved similar mechanisms for breaching the cell membrane.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Electron Microscopy Data Bank
Protein Data Bank
Data deposits
The atomic coordinates and structure factor files have been deposited into the Protein Data Bank (PDB) under accession numbers 5FB4, 5FB5 and 5FEI. The electron microscopy maps have been deposited into the Electron Microscopy Data Bank (EMDB) under accession numbers EMD-6556, EMD-6557 and EMD-6558.
References
Ackermann, H. W. Bacteriophage observations and evolution. Res. Microbiol. 154, 245–251 (2003)
Panjwani, A. et al. Capsid protein VP4 of human rhinovirus induces membrane permeability by the formation of a size-selective multimeric pore. PLoS Pathog. 10, e1004294 (2014)
Shukla, A., Padhi, A. K., Gomes, J. & Banerjee, M. The VP4 peptide of hepatitis A virus ruptures membranes through formation of discrete pores. J. Virol. 88, 12409–12421 (2014)
Galloux, M. et al. NMR structure of a viral peptide inserted in artificial membranes: a view on the early steps of the birnavirus entry process. J. Biol. Chem. 285, 19409–19421 (2010)
Tao, Y. et al. Assembly of a tailed bacterial virus and its genome release studied in three dimensions. Cell 95, 431–437 (1998)
Anderson, D. L. & Reilly, B. E. in Bacillus subtilis and Other Gram-Positive Bacteria: Biochemistry, Physiology, and Molecular Genetics (eds Sonenshein, A. L., Hoch, J. A. & Losick, R. ) 859–867 (American Society for Microbiology, 1993)
Morais, M. C. et al. Conservation of the capsid structure in tailed dsDNA bacteriophages: the pseudoatomic structure of ϕ29. Mol. Cell 18, 149–159 (2005)
Meijer, W. J., Horcajadas, J. A. & Salas, M. ϕ29 family of phages. Microbiol. Mol. Biol. Rev. 65, 261–287 (2001)
Xiang, Y. et al. Structural changes of bacteriophage ϕ29 upon DNA packaging and release. EMBO J. 25, 5229–5239 (2006)
Tang, J. et al. DNA poised for release in bacteriophage ϕ29. Structure 16, 935–943 (2008)
Xiang, Y. et al. Crystal and cryoEM structural studies of a cell wall degrading enzyme in the bacteriophage ϕ29 tail. Proc. Natl Acad. Sci. USA 105, 9552–9557 (2008)
Xiang, Y. et al. Crystallographic insights into the autocatalytic assembly mechanism of a bacteriophage tail spike. Mol. Cell 34, 375–386 (2009)
Cohen, D. N. et al. Shared catalysis in virus entry and bacterial cell wall depolymerization. J. Mol. Biol. 387, 607–618 (2009)
Cohen, D. N., Erickson, S. E., Xiang, Y., Rossmann, M. G. & Anderson, D. L. Multifunctional roles of a bacteriophage ϕ29 morphogenetic factor in assembly and infection. J. Mol. Biol. 378, 804–817 (2008)
Cardarelli, L. et al. Phages have adapted the same protein fold to fulfill multiple functions in virion assembly. Proc. Natl Acad. Sci. USA 107, 14384–14389 (2010)
Wriggers, W. Using Situs for the integration of multi-resolution structures. Biophys. Rev. 2, 21–27 (2010)
Remmert, M., Biegert, A., Hauser, A. & Söding, J . HHblits: lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat. Methods 9, 173–175 (2011)
Johnson, J. E. & Vogt, P. K. Cell entry by non-enveloped viruses. Curr. Top. Microbiol. Immunol. 343, v–vii (2010)
Doan, T. et al. FisB mediates membrane fission during sporulation in Bacillus subtilis . Genes Dev. 27, 322–334 (2013)
Xiang, Y. & Rossmann, M. G. Structure of bacteriophage ϕ29 head fibers has a supercoiled triple repeating helix-turn-helix motif. Proc. Natl Acad. Sci. USA 108, 4806–4810 (2011)
Song, Y. et al. High-resolution comparative modeling with RosettaCM. Structure 21, 1735–1742 (2013)
Joseph, P., Fantino, J. R., Herbaud, M. L. & Denizot, F. Rapid orientated cloning in a shuttle vector allowing modulated gene expression in Bacillus subtilis . FEMS Microbiol. Lett. 205, 91–97 (2001)
Jarmer, H., Berka, R., Knudsen, S. & Saxild, H. H. Transcriptome analysis documents induced competence of Bacillus subtilis during nitrogen limiting conditions. FEMS Microbiol. Lett. 206, 197–200 (2002)
Llaguno, M. C. et al. Chemically functionalized carbon films for single molecule imaging. J. Struct. Biol. 185, 405–417 (2014)
Ludtke, S. J. 3-D structures of macromolecules using single-particle analysis in EMAN. Methods Mol. Biol. 673, 157–173 (2010)
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012)
Trabuco, L. G., Villa, E., Schreiner, E., Harrison, C. B. & Schulten, K. Molecular dynamics flexible fitting: a practical guide to combine cryo-electron microscopy and X-ray crystallography. Methods 49, 174–180 (2009)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008)
Bricogne, G., Vonrhein, C., Flensburg, C., Schiltz, M. & Paciorek, W. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr. D 59, 2023–2030 (2003)
Cowtan, K. D., Zhang, K. Y. J. & Main, P. in International Tables for Crystallography Vol. F (eds Rossmann, M. G. & Arnold, E. ) 507–510 (Kluwer Academic Publishers, 2000)
Rossmann, M. G. & Blow, D. M. The detection of sub-units within the crystallographic asymmetric unit. Acta Crystallogr. 15, 24–31 (1962)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Gouet, P., Courcelle, E., Stuart, D. I. & Métoz, F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15, 305–308 (1999)
Ishida, T. & Kinoshita, K. PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res. 35, W460–W464 (2007)
Acknowledgements
We thank L. Q. Zhang, N. Yan, H. T. Li, S. L. Fan, N. Gao, C. Z. Zhou, D. L. Anderson and M. G. Rossmann for support; the Tsinghua University Branch of the China National Center for Protein Sciences for the facility support; and the staff at the Shanghai Synchrotron Research Facility beam line BL17U for assistance with data collection. This work was supported by funds from the 973 program (2015CB910102), the National Natural Science Foundation of China (31470721 and 81550001), the Junior Thousand Talents Program of China (20131770418) and the Beijing Advanced Innovation Center for Structural Biology to Y.X.
Author information
Authors and Affiliations
Contributions
J.X. and Y.X. designed the research; J.X., M.G., D.W. and Y.X. performed the experiments; J.X. and Y.X. analysed the data and wrote the paper; and all authors contributed to the editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Mass spectrometry and sequence analysis showing the disordered region of gp9.
a, Western blotting and mass spectrometry analysis of the recombinant full-length and degraded gp9 proteins. Positions of the full-length and degraded gp9 proteins are indicated with arrows and labelled with P5–P1 in the western blotting image. Corresponding peaks in the mass spectra are labelled in the same order as in the western blot. b, Disordered region analysis of gp9 using the online tool ProDOS37. The threshold (red line) was set based on a false positive rate of 0.15. Residues with a disorder probability of more than 0.38 are considered disordered. The start and end positions of several predicted long disordered regions are indicated with red arrows. c, Diagrams showing the location of the predicted disordered region in the sequence and the fragments determined by the mass spectrometry analysis.
Extended Data Figure 2 Surface charge distribution of gp9.
a, Diagram showing the surface charge distribution of gp9Δ417–491. Negative and positive electrostatic potentials are coloured red and blue, respectively. The monomers within the hexamer interact with each other primarily through hydrogen bonds and hydrophobic interactions. The outer cylindrical surface of the tube is hydrophilic. The top and bottom surfaces of the tube have substantially different features. The top surface on the N-β-barrel domain side is largely hydrophobic, whereas the bottom surface on the tip β-domain side is rich in negatively charged residues. b, Diagram showing the surface charge distribution of the interior L loops. Negative and positive electrostatic potentials are coloured as in a. A thin central slice of the tube is shown and coloured dark grey to indicate the tube boundary.
Extended Data Figure 3 Fitted gp9 structure in a cryo-EM map of the tail.
Diagram showing the gp9 hexamer structure fitted in a cryo-EM map of the tail contoured at 4.0σ. An ~45-Å-thick cross-section is shown. The gp9 hexamer structure is shown as a ribbon representation with the L loops coloured red. The density map is shown as a solid surface representation and coloured semi-transparent grey.
Extended Data Figure 4 Electron microscopy images showing the mature ϕ29–liposome complex.
a, Cryo-EM images of mature ϕ29 particles with B. subtilis cell membrane-like liposomes at neutral pH. b, Negatively stained electron microscopy images showing the low-pH-treated, DNA-emptied ϕ29 particles aggregating around a liposome that contains lipids similar to those in the eukaryotic cell membrane. Scale bars, 200 nm.
Extended Data Figure 5 Release of calcein from liposomes induced by ϕ29.
Time-course release of calcein from liposomes induced by ϕ29 in a low- or neutral-pH buffer is shown in solid lines with blue (low pH) or red (neutral pH) triangles representing the data points. Percentages of the calcein released from the liposomes were calculated from (Fd − F0)/(Fmax − F0) where Fd is the measured fluorescence value of the low or neutral pH sample, F0 is the background fluorescence and Fmax is the maximum fluorescence value measured after adding Triton X-100 at a final concentration of 0.02% (v/v). F0 (the x axis) is generated from a linear least square fitting of the fluorescence values measured on a liposome–low-pH buffer mixture without adding the phage. The data used for the linear least square fitting of the background fluorescence are represented by green triangles. The data are expressed as the mean ± s.d. of three independent measurements.
Extended Data Figure 6 Structure models of the pre- and post-DNA-ejection L loops.
a, Post-DNA-ejection L loop structure modelled with RosettaCM using the electron microscopy map as a restraint and fitted into the post-DNA-ejection tail electron microscopy map. The gp9 structure is shown in a ribbon representation. The L loops are coloured red. The rest of the structure is coloured grey. b, Structural comparison of the pre- and post-DNA-ejection L loop structures. Left, pre-DNA-ejection gp9 structure shown in a ribbon representation with the L loops coloured red. Right, post-DNA-ejection gp9 structure shown in a ribbon representation with the modelled structure of the flexible region coloured cyan and the rest of the L loop coloured red.
Extended Data Figure 7 Sequence alignments of the gp9 L-loop-like peptides in phage tail proteins.
a, Sequence alignment of the gp9 L-loop and L-loop-like peptides from short non-contractile tails. Conserved residues are boxed and coloured red. The core hydrophobic region of the L loop is indicated by a dashed box. b, Sequence alignment of gp9 L loop, HIV fusion peptide, influenza fusion peptide and a potential hydrophobic membrane active peptide of the bacteriophage T4 tail protein gp5. Conserved residues are boxed and coloured red. Completely conserved residues are shown in white on a red background. The core region of the HIV fusion peptide is indicated by a dashed box. c, Schematic diagram showing a possible mechanism for the exposure of the potential T4 hydrophobic membrane active peptide during infection. The structure of the T4 gp27 (top β-barrels) and gp5 (bottom) complex is shown in ribbon representation. The potential hydrophobic peptide of gp5 is coloured red. The rest of the complex structure is coloured grey. The potential hydrophobic peptide is exposed after the release of the gp5 C-terminal needle. Conformational changes of the lysozyme domain trigger the insertion of the hydrophobic peptide into the membrane.
Rights and permissions
About this article
Cite this article
Xu, J., Gui, M., Wang, D. et al. The bacteriophage ϕ29 tail possesses a pore-forming loop for cell membrane penetration. Nature 534, 544–547 (2016). https://doi.org/10.1038/nature18017
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18017
This article is cited by
-
Structural assembly of the tailed bacteriophage ϕ29
Nature Communications (2019)
-
Targeting mechanisms of tailed bacteriophages
Nature Reviews Microbiology (2018)
-
Artificial bio-nanomachines based on protein needles derived from bacteriophage T4
Biophysical Reviews (2018)
-
Lipid environment of membrane proteins in cryo-EM based structural analysis
Biophysical Reviews (2018)
-
Bacteriophage T5 tail tube structure suggests a trigger mechanism for Siphoviridae DNA ejection
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.