Abstract
The epidermal growth factor receptor (EGFR)-directed tyrosine kinase inhibitors (TKIs) gefitinib, erlotinib and afatinib are approved treatments for non-small cell lung cancers harbouring activating mutations in the EGFR kinase1,2, but resistance arises rapidly, most frequently owing to the secondary T790M mutation within the ATP site of the receptor3,4. Recently developed mutant-selective irreversible inhibitors are highly active against the T790M mutant5,6, but their efficacy can be compromised by acquired mutation of C797, the cysteine residue with which they form a key covalent bond7. All current EGFR TKIs target the ATP-site of the kinase, highlighting the need for therapeutic agents with alternative mechanisms of action. Here we describe the rational discovery of EAI045, an allosteric inhibitor that targets selected drug-resistant EGFR mutants but spares the wild-type receptor. The crystal structure shows that the compound binds an allosteric site created by the displacement of the regulatory C-helix in an inactive conformation of the kinase. The compound inhibits L858R/T790M-mutant EGFR with low-nanomolar potency in biochemical assays. However, as a single agent it is not effective in blocking EGFR-driven proliferation in cells owing to differential potency on the two subunits of the dimeric receptor, which interact in an asymmetric manner in the active state8. We observe marked synergy of EAI045 with cetuximab, an antibody therapeutic that blocks EGFR dimerization9,10, rendering the kinase uniformly susceptible to the allosteric agent. EAI045 in combination with cetuximab is effective in mouse models of lung cancer driven by EGFR(L858R/T790M) and by EGFR(L858R/T790M/C797S), a mutant that is resistant to all currently available EGFR TKIs. More generally, our findings illustrate the utility of purposefully targeting allosteric sites to obtain mutant-selective inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009)
Yu, H. A. & Pao, W. Targeted therapies: Afatinib—new therapy option for EGFR-mutant lung cancer. Nat. Rev. Clin. Oncol. 10, 551–552 (2013)
Gainor, J. F. & Shaw, A. T. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J. Clin. Oncol. 31, 3987–3996 (2013)
Chong, C. R. & Jänne, P. A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 19, 1389–1400 (2013)
Walter, A. O. et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 3, 1404–1415 (2013)
Finlay, M. R. et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J. Med. Chem. 57, 8249–8267 (2014)
Thress, K. S. et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 21, 560–562 (2015)
Zhang, X., Gureasko, J., Shen, K., Cole, P. A. & Kuriyan, J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125, 1137–1149 (2006)
Goldstein, N. I., Prewett, M., Zuklys, K., Rockwell, P. & Mendelsohn, J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1, 1311–1318 (1995)
Li, S. et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7, 301–311 (2005)
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004)
Pao, W. et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA 101, 13306–13311 (2004)
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004)
Yun, C. H. et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl Acad. Sci. USA 105, 2070–2075 (2008)
Zhou, W. et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 462, 1070–1074 (2009)
Cross, D. A. et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 4, 1046–1061 (2014)
Sequist, L. V. et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med. 372, 1700–1709 (2015)
Jänne, P. A. et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 372, 1689–1699 (2015)
Ercan, D. et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin. Cancer Res. 21, 3913–3923 (2015)
Tsou, H. R. et al. Optimization of 6,7-disubstituted-4-(arylamino)quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. J. Med. Chem. 48, 1107–1131 (2005)
Wood, E. R. et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 64, 6652–6659 (2004)
Zhao, Y. & Adjei, A. A. The clinical development of MEK inhibitors. Nat. Rev. Clin. Oncol. 11, 385–400 (2014)
Yun, C. H. et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11, 217–227 (2007)
Red Brewer, M. et al. Mechanism for activation of mutated epidermal growth factor receptors in lung cancer. Proc. Natl Acad. Sci. USA 110, E3595–E3604 (2013)
Shan, Y. et al. Oncogenic mutations counteract intrinsic disorder in the EGFR kinase and promote receptor dimerization. Cell 149, 860–870 (2012)
Cho, J. et al. Cetuximab response of lung cancer-derived EGF receptor mutants is associated with asymmetric dimerization. Cancer Res. 73, 6770–6779 (2013)
Li, D. et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell 12, 81–93 (2007)
Oxnard, G. R. et al. in 16th World Conference on Lung Cancer (Denver, Colorado, 2015)
Hong, L., Quinn, C. M. & Jia, Y. Evaluating the utility of the HTRF Transcreener ADP assay technology: a comparison with the standard HTRF assay technology. Anal. Biochem. 391, 31–38 (2009)
Engelman, J. A. et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc. Natl Acad. Sci. USA 102, 3788–3793 (2005)
Beard, C., Hochedlinger, K., Plath, K., Wutz, A. & Jaenisch, R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis 44, 23–28 (2006)
Muller, G. W. Cyclic amides. US patent 5698579 (1997)
Acknowledgements
This work was supported in part by NIH grants CA116020 (M.J.E.), CA154303 (M.J.E., K.-K.W. and P.A.J.), CA120964 (K.-K.W.) and CA135257 (P.A.J.), and by the Gross-Loh Family Fund for Lung Cancer Research (K.-K.W.). We thank N. Gray for helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
M.J.E., P.A.J., K.-K.W., Y.J., G.L., P.-Y.M., J.H., and S.B. coordinated the study. Y.J., M.M., J. Juarez, M.D., B.B., E.P., C.-H.Y., D.E., C.X., K.R., T.C., H.Z., S.P., and J. Jang designed and performed experiments. Y.J., M.M., J. Juarez, M.D., B.B., S.B., E.P., C.-H.Y., D.E., C.X., K.R., M.J.E., P.J., and K.-K.W. interpreted data. M.M., J. Juarez, M.D., G.L., P.-Y.M., R.E., T.H.M, M.M., C-H.Y. and W.L. prepared reagents. Y.J., K.-K.W., P.A.J. and M.J.E. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.-K.W. has an equity interest in G1 Therapeutics and Gatekeeper Pharmaceuticals, and has sponsored research agreements with AstraZeneca and Gilead Pharmaceuticals. P.A.J. receives sponsored research support from AstraZeneca; has ownership interest (including patents) in Gatekeeper Pharmaceuticals; is a consultant/advisory board member for AstraZeneca, Boehringer Ingelheim, Chugai Pharmaceuticals, Clovis Oncology, Genentech, Merrimack, Pfizer, and Sanofi. M.J.E. is a consultant for and receives sponsored research support from Novartis Institutes for Biomedical Research.
Extended data figures and tables
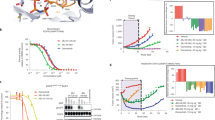
Extended Data Figure 1 Inhibition of wild-type and mutant EGFR kinases by EAI001 and EAI045 in purified enzyme assays.
a, Inhibition of wild-type and mutant EGFR kinases by EAI001. Activity of the indicated mutant EGFR kinase (residues 696–1022) was measured in the presence of increasing concentrations of EAI001. The HTRF assay was carried out using either 1 μM ATP (left) or 1 mM ATP (right). b, Inhibition of EGFR(L858R/T790M) by EAI045 (left) or erlotinib (right) at a range of ATP concentrations, as indicated. Assay was performed using an HTRF-based assay as described in the Methods. Error bars indicate s.d. (n = 2).
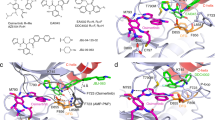
Extended Data Figure 2 Comparison of the binding site of EGFR allosteric inhibitors with those of lapatinib and allosteric MEK inhibitors.
a, Structure of EAI001 in complex with EGFR for comparison. b, Structure of MEK1 kinase bound to allosteric inhibitor GDC0973 (PDB, 4AN2). GDC0973 (also called XL518, cobimetinib) and other allosteric MEK inhibitors occupy a pocket created by displacement of the C-helix in the inactive conformation of the kinase. Most allosteric MEK inhibitors make hydrogen-bond interactions with the γ-phosphate group of ATP that are important for their potency. The allosteric EGFR inhibitors we describe here bind in a generally analogous location in EGFR, but lack any clear structural similarity to MEK inhibitors and do not contact the γ-phosphate group of ATP. c, The structure of lapatinib bound to EGFR (PDB, 1XKK). Both lapatinib and neratinib (see Fig. 1d) bind an inactive conformation of the kinase. Like gefitinib and erlotinib, both occupy the ATP site, but also extend into the allosteric pocket occupied by EAI001. Note that like neratinib, lapatinib places aromatic phenyl or pyridinyl groups in positions similar to those occupied by the aminothiazole and phenyl substituents of EAI001.
Extended Data Figure 3 EAI001 binding is incompatible with the inactive conformation of wild-type EGFR.
Superposition of the EAI001-bound EGFR structure reported here with the structure of wild-type EGFR kinase in the inactive conformation (grey, PDB, 2GS7). EAI001 (shown with carbon atoms in green) clashes with the side chains of leucines 858 and 861 in the wild-type EGFR structure. These leucine residues lie in a short helical segment at the N terminus of the activation loop. The L858R substitution disrupts this helix. We propose that this effect explains, in part, the selectivity of the allosteric inhibitor for the L858R/T790M mutant. Note that EAI001 was crystallized with the EGFR(T790M/V948R), as we were unable to obtain crystals with the L858R/T790M or L858R/T790M/V948R proteins. The compound induces unstructuring of the activation loop helix and repositions L858, which is in contact with the 1-oxoisoindolinyl group of the inhibitor. The location and conformation of the inhibitor is expected to be the same in the context of the L858R mutation, but the details of the interaction with this portion of the activation loop will necessarily differ due to the mutation.
Extended Data Figure 4 Cellular activity of EAI045.
a, EAI045 inhibition of EGFR(L858R/T790M) in NIH-3T3 cells. Western blotting with the indicated concentrations of the allosteric inhibitor or with 1 μM WZ4002 as control (WZ) was carried out 6 h after compound addition. b–e, Profiling of EAI045 in Ba/F3 models bearing mutant EGFR or the parental Ba/F3 cell line, as indicted. Inhibition by WZ4002 is shown as a positive control. For gel source data, see Supplementary Fig. 1.
Extended Data Figure 5 Cellular and in vivo efficacy of EAI045 in combination with cetuximab.
a, Ba/F3 cells bearing EGFR(L858R/T790M/C797S) were treated with EAI045 alone or with EAI045 plus cetuximab and proliferation was measured using the MTS assay after 72 h. b, MRI imaging of cohorts L858R/T790M, exon19del/T790M, and L858R/T790M/C797S genetically engineered EGFR-mutant mice before treatment and 1 or 2 weeks after treatment with EAI045 and cetuximab. These cohorts of tumour bearing mice were used for short term efficacy and pharmacodynamic studies, and are distinct from those used for the tumour volume measurements shown in Fig. 3.
Supplementary information
Supplementary Figures 1 and 3
Supplementary Figure 1 shows the source gel data for Figures 2a, 3d, 3e, and Extended Data Figure 4a. Supplementary Figure 3 contains a description of generation and characterization of LSL-EGFR T790M/L858R/C797S conditional transgenic mice. (PDF 2228 kb)
Supplementary Figure 2
This file contains the source data for tumor volume measurements in Figure 3a-c. (PDF 9532 kb)
Rights and permissions
About this article
Cite this article
Jia, Y., Yun, CH., Park, E. et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 534, 129–132 (2016). https://doi.org/10.1038/nature17960
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17960
This article is cited by
-
Synthesis and preclinical evaluation of [11C]EAI045 as a PET tracer for imaging tumors expressing mutated epidermal growth factor receptor
EJNMMI Research (2024)
-
Oncogenic alterations in advanced NSCLC: a molecular super-highway
Biomarker Research (2024)
-
Epigenetic-based combination therapy and liposomal codelivery overcomes osimertinib-resistant NSCLC via repolarizing tumor-associated macrophages
Acta Pharmacologica Sinica (2024)
-
Toward the next generation EGFR inhibitors: an overview of osimertinib resistance mediated by EGFR mutations in non-small cell lung cancer
Cell Communication and Signaling (2023)
-
PROTAC’ing oncoproteins: targeted protein degradation for cancer therapy
Molecular Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.