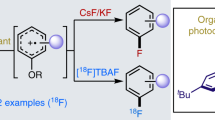
Abstract
Nucleophilic aromatic substitution (SNAr) is widely used by organic chemists to functionalize aromatic molecules, and it is the most commonly used method to generate arenes that contain 18F for use in positron-emission tomography (PET) imaging1. A wide range of nucleophiles exhibit SNAr reactivity, and the operational simplicity of the reaction means that the transformation can be conducted reliably and on large scales2. During SNAr, attack of a nucleophile at a carbon atom bearing a ‘leaving group’ leads to a negatively charged intermediate called a Meisenheimer complex. Only arenes with electron-withdrawing substituents can sufficiently stabilize the resulting build-up of negative charge during Meisenheimer complex formation, limiting the scope of SNAr reactions: the most common SNAr substrates contain strong π-acceptors in the ortho and/or para position(s)3. Here we present an unusual concerted nucleophilic aromatic substitution reaction (CSNAr) that is not limited to electron-poor arenes, because it does not proceed via a Meisenheimer intermediate. We show a phenol deoxyfluorination reaction for which CSNAr is favoured over a stepwise displacement. Mechanistic insights enabled us to develop a functional-group-tolerant 18F-deoxyfluorination reaction of phenols, which can be used to synthesize 18F-PET probes. Selective 18F introduction, without the need for the common, but cumbersome, azeotropic drying of 18F, can now be accomplished from phenols as starting materials, and provides access to 18F-labelled compounds not accessible through conventional chemistry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Data deposits
Atomic coordinates and structure factors for the reported crystal structures have been deposited in the Cambridge Crystallographic Data Centre under accession number CCDC-1419728.
References
Fernández, I., Frenking, G. & Uggerud, E. Rate-determining factors in nucleophilic aromatic substitution reactions. J. Org. Chem. 75, 2971–2980 (2010)
Terrier, F. Modern Nucleophilic Aromatic Substitution 1–94 (Wiley, 2013)
Chéron, N., El Kaïm, L., Grimaud, L. & Fleurat-Lessard, P. Evidences for the key role of hydrogen bonds in nucleophilic aromatic substitution reactions. Chemistry 17, 14929–14934 (2011)
Picazo, E., Houk, K. N. & Garg, N. K. Computational predictions of substituted benzyne and indolyne regioselectivities. Tetrahedr. Lett. 56, 3511–3514 (2015)
Crampton, M. R. in Organic Reaction Mechanisms—2010: An Annual Survey Covering the Literature Dated January to December 2010 (ed. Knipe, A. C. ) 175–190 (Wiley, 2012)
Lloyd-Jones, G. C., Moseley, J. D. & Renny, J. S. Mechanism and application of the Newman-Kwart O→S rearrangement of O-aryl thiocarbamates. Synthesis 2008, 661–689 (2008)
Tang, P., Wang, W. & Ritter, T. Deoxyfluorination of phenols. J. Am. Chem. Soc. 133, 11482–11484 (2011)
Fujimoto, T., Becker, F. & Ritter, T. PhenoFluor: practical synthesis, new formulation, and deoxyfluorination of heteroaromatics. Org. Process Res. Dev. 18, 1041–1044 (2014)
Glukhovtsev, M. N., Bach, R. D. & Laiter, S. Single-step and multistep mechanisms of aromatic nucleophilic substitution of halobenzenes and halonitrobenzenes with halide anions: ab initio computational study. J. Org. Chem. 62, 4036–4046 (1997)
Fry, S. E. & Pienta, N. J. Effects of molten salts on reactions. Nucleophilic aromatic substitution by halide ions in molten dodecyltributylphosphonium salts. J. Am. Chem. Soc. 107, 6399–6400 (1985)
Hunter, A. et al. Stepwise versus concerted mechanisms at trigonal carbon: transfer of the 1,3,5-triazinyl group between aryl oxide ions in aqueous solution. J. Am. Chem. Soc. 117, 5484–5491 (1995)
Renfrew, A. H. M., Taylor, J. A., Whitmore, J. M. J. & Williams, A. A single transition state in nucleophilic aromatic substitution: reaction of phenolate ions with 2-(4-nitrophenoxy)-4,6-dimethoxy-1,3,5-triazine in aqueous solution. J. Chem. Soc. Perkin Trans. 2 1993, 1703–1704 (1993)
Dub, P. A. et al. C–F bond breaking through aromatic nucleophilic substitution with a hydroxo ligand mediated via water bifunctional activation. Bull. Chem. Soc. Jpn 86, 557–568 (2013)
Goryunov, L. I. et al. Di- and trifluorobenzenes in reactions with Me2EM (E = P, N; M = SiMe3, SnMe3, Li) reagents: evidence for a concerted mechanism of aromatic nucleophilic substitution. Eur. J. Org. Chem. 2010, 1111–1123 (2010)
Nawaz, F. et al. Temporary intramolecular generation of pyridine carbenes in metal-free three-component C–H bond functionalisation/aryl-transfer reactions. Chemistry 19, 17578–17583 (2013)
Renfrew, A. H. M., Taylor, J. A., Whitmore, J. M. J. & Williams, A. Timing of bonding changes in fundamental reactions in solutions: pyridinolysis of a triazinylpyridinium salt. J. Chem. Soc. Perkin Trans. 2 1994, 2383–2384 (1994)
Williams, A. The diagnosis of concerted organic mechanisms. Chem. Soc. Rev. 23, 93–100 (1994)
Sawyer, C. B. & Kirsch, J. F. Kinetic isotope effects for reactions of methyl formate-methoxyl-18O. J. Am. Chem. Soc. 95, 7375–7381 (1973)
Lasne, M.-C. et al. in Contrast Agents II Vol. 222 (ed. Krause, W. ) Ch. 7, 201–258 (Springer, 2002)
Matsson, O. & MacMillar, S. Isotope effects for fluorine-18 and carbon-11 in the study of reaction mechanisms. J. Labelled Comp. Radiopharm. 50, 982–988 (2007)
Yamabe, S., Minato, T. & Kawabata, Y. The importance of the σ*–π* orbital mixing for the nucleophilic displacement on the unsaturated carbon. Can. J. Chem. 62, 235–240 (1984)
Mu, L. et al. 18F-radiolabeling of aromatic compounds using triarylsulfonium salts. Eur. J. Org. Chem. 2012, 889–892 (2012)
Sun, H. & DiMagno, S. G. Room-temperature nucleophilic aromatic fluorination: experimental and theoretical studies. Angew. Chem. Int. Ed. 45, 2720–2725 (2006)
Neumann, C. N. & Ritter, T. Late-stage fluorination: fancy novelty or useful tool? Angew. Chem. Int. Ed. 54, 3216–3221 (2015)
Tredwell, M. et al. A general copper-mediated nucleophilic 18F fluorination of arenes. Angew. Chem. Int. Ed. 53, 7751–7755 (2014)
Gao, Z. et al. Metal-free oxidative fluorination of phenols with [18F]fluoride. Angew. Chem. Int. Ed. 51, 6733–6737 (2012)
Ichiishi, N. et al. Copper-catalyzed [18F]fluorination of (mesityl)(aryl)iodonium salts. Org. Lett. 16, 3224–3227 (2014)
Lee, E., Hooker, J. M. & Ritter, T. Nickel-mediated oxidative fluorination for PET with aqueous [18F]fluoride. J. Am. Chem. Soc. 134, 17456–17458 (2012)
Lee, E. et al. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science 334, 639–642 (2011)
Bronner, S. M., Goetz, A. E. & Garg, N. K. Overturning indolyne regioselectivities and synthesis of indolactam V. J. Am. Chem. Soc. 133, 3832–3835 (2011)
Acknowledgements
We thank the Patty and Michael Phelps Foundation and the National Institutes of Health (NIH) National Institute of General Medical Sciences (GM088237) for funding. Radioisotope production and use were enabled by a shared instrument grant from the NIH (1S10RR017208). We thank S. Arlow and C. Kleinlein for preliminary mechanistic work and H. Lee for assistance with X-ray crystallography. We thank N. A. Stephenson for a synthetic precursor for S6 as well as the 19F-standard for this substrate.
Author information
Authors and Affiliations
Contributions
C.N.N. designed and performed the experiments, with input from T.R. and J.M.H. C.N.C. analysed the data. T.R. directed the project. C.N.N. and T.R. prepared the manuscript with input from J.M.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Figures 1-47, Supplementary Tables 1-5 and Supplementary References – see contents pages for full details. (PDF 10669 kb)
Rights and permissions
About this article
Cite this article
Neumann, C., Hooker, J. & Ritter, T. Concerted nucleophilic aromatic substitution with 19F− and 18F−. Nature 534, 369–373 (2016). https://doi.org/10.1038/nature17667
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17667
This article is cited by
-
State of the art procedures towards reactive [18F]fluoride in PET tracer synthesis
EJNMMI Radiopharmacy and Chemistry (2023)
-
Radiochemistry for positron emission tomography
Nature Communications (2023)
-
Deoxyfluorination of phenols for chemoselective 18F-labeling of peptides
Nature Protocols (2023)
-
Arene radiofluorination enabled by photoredox-mediated halide interconversion
Nature Chemistry (2022)
-
Closing the gap between 19F and 18F chemistry
EJNMMI Radiopharmacy and Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.