Abstract
Blood vessels define local microenvironments in the skeletal system, play crucial roles in osteogenesis and provide niches for haematopoietic stem cells1,2,3,4,5,6. The properties of niche-forming vessels and their changes in the ageing organism remain incompletely understood. Here we show that Notch signalling in endothelial cells leads to the expansion of haematopoietic stem cell niches in bone, which involves increases in CD31-positive capillaries and platelet-derived growth factor receptor-β (PDGFRβ)-positive perivascular cells, arteriole formation and elevated levels of cellular stem cell factor. Although endothelial hypoxia-inducible factor signalling promotes some of these changes, it fails to enhance vascular niche function because of a lack of arterialization and expansion of PDGFRβ-positive cells. In ageing mice, niche-forming vessels in the skeletal system are strongly reduced but can be restored by activation of endothelial Notch signalling. These findings indicate that vascular niches for haematopoietic stem cells are part of complex, age-dependent microenvironments involving multiple cell populations and vessel subtypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
14 September 2016
Nature 532, 380–384 (2016); doi:10.1038/nature17638 In this Letter, the graph for the Fbxw7 mutants in Fig. 2e was a duplicate of the NICD graph. This inadvertently occurred during the reformatting of the accepted manuscript, and the panel has now been corrected in Fig. 1 of this Corrigendum, but has not been corrected in the online versions of the original paper.
References
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012)
Hooper, A. T. et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274 (2009)
Kiel, M. J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005)
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014)
Acar, M. et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130 (2015)
Ramasamy, S. K., Kusumbe, A. P., Wang, L. & Adams, R. H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376–380 (2014)
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 (2013)
Davy, A., Bush, J. O. & Soriano, P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 4, e315 (2006)
Adams, R. H. et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 13, 295–306 (1999)
Corada, M. et al. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nature Commun. 4, 2609 (2013)
Adams, R. H. & Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nature Rev. Mol. Cell Biol. 8, 464–478 (2007)
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 6, 389–395 (2000)
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010)
Hellström, M., Kalén, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999)
Chambers, S. M. et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 5, e201 (2007)
Morrison, S. J., Wandycz, A. M., Akashi, K., Globerson, A. & Weissman, I. L. The aging of hematopoietic stem cells. Nature Med. 2, 1011–1016 (1996)
Prisby, R. D. et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J. Bone Miner. Res. 22, 1280–1288 (2007)
Moerman, E. J., Teng, K., Lipschitz, D. A. & Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 3, 379–389 (2004)
Zsebo, K. M. et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 63, 213–224 (1990)
Vooijs, M. et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development 134, 535–544 (2007)
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007)
Wu, G. et al. SEL-10 is an inhibitor of notch signaling that targets notch for ubiquitin-mediated protein degradation. Mol. Cell. Biol. 21, 7403–7415 (2001)
Roca, C. & Adams, R. H. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 21, 2511–2524 (2007)
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003)
Red-Horse, K., Ueno, H., Weissman, I. L. & Krasnow, M. A. Coronary arteries form by developmental reprogramming of venous cells. Nature 464, 549–553 (2010)
Xu, C. et al. Arteries are formed by vein-derived endothelial tip cells. Nature Commun. 5, 5758 (2014)
Kinashi, T. & Springer, T. A. Steel factor and c-kit regulate cell-matrix adhesion. Blood 83, 1033–1038 (1994)
Miyazawa, K. et al. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood 85, 641–649 (1995)
Poulos, M. G. et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Reports 4, 1022–1034 (2013)
Dykstra, B., Olthof, S., Schreuder, J., Ritsema, M. & de Haan, G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J. Exp. Med. 208, 2691–2703 (2011)
Han, H. et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637–645 (2002)
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010)
Hoeck, J. D. et al. Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nature Neurosci. 13, 1365–1372 (2010)
Koch, U. et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J. Exp. Med. 205, 2515–2523 (2008)
Tang, N. et al. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6, 485–495 (2004)
Haase, V. H., Glickman, J. N., Socolovsky, M. & Jaenisch, R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc. Natl Acad. Sci. USA 98, 1583–1588 (2001)
Murtaugh, L. C., Stanger, B. Z., Kwan, K. M. & Melton, D. A. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl Acad. Sci. USA 100, 14920–14925 (2003)
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010)
Kisanuki, Y. Y. et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo . Dev. Biol. 230, 230–242 (2001)
Itkin, T. et al. FGF-2 expands murine hematopoietic stem and progenitor cells via proliferation of stromal cells, c-Kit activation, and CXCL12 down-regulation. Blood 120, 1843–1855 (2012)
Miller, C. L., Dykstra, B. & Eaves, C. J. Characterization of mouse hematopoietic stem and progenitor cells. Curr. Protoc. Immunol. 22, 22B.2.1–22B.2.31 (2008)
Gomez, C. R., Knutson, G. J., Clifton, K. B., Schreiber, C. A. & Vuk-Pavlović, S. Age-dependent response of murine female bone marrow cells to hyperbaric oxygen. Biogerontology 13, 287–297 (2012)
Acknowledgements
We thank M. Schiller for technical assistance, A. Starsichova for help with bone sample processing, M. Stehling for fluorescence-activated cell sorting (FACS), S. Volkery for microscopy, and M. Vanlandewijck and K. Nahar for help with PdgfbiOE-EC bones. Funding was provided by the Max Planck Society, the University of Münster, the DFG cluster of excellence ‘Cells in Motion’, and the European Research Council (AdG 339409 ‘AngioBone’). This research was partly supported by a joint grant from the Ministry of Science, Technology & Space, Israel, DKFZ Germany, ERC AdG 294556 ‘BBBarrier’, the Knut and Alice Wallenberg Foundation and the Swedish Cancer Foundation.
Author information
Authors and Affiliations
Contributions
A.P.K., S.K.R. and R.H.A. designed experiments and interpreted results. A.P.K. and S.K.R. organized and conducted most experiments, including generation and characterization of mouse lines, imaging, flow cytometric analysis and transplantations. T.I. and T.L. designed and performed transplantation experiments. M.A.M. and C.B. generated and provided samples from PdgfbiOE-EC mice. A.P.K and U.H.L. analysed Efnb2 and NICD-Cre mice. A.P.K., S.K.R. and R.H.A. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Summary of key findings and analysis of vessel subtypes.
a, Type H vessels express arterial markers and, unlike type L (sinusoidal) vessels, make direct connections to distal arterioles. PDGFRβ+ perivascular cells (PVC) are selectively abundant around type H vessels and endosteal arteries. In addition, HSCs are frequently detected near the type H endothelium and arterioles in the endosteal region. Endothelial Notch signalling drives artery formation, expansion of type H ECs and PDGFRβ+ PVCs, production of cell-bound SCF and thereby enhances vascular HSC niche function. By contrast, endothelial HIF signalling fails to induce artery formation and expansion of PDGFRβ+ PVCs, which does not result in more HSC niches despite of increases in type H ECs and Osterix+ osteoprogenitor (OP) cells. b, Confocal images of 8-week-old tibial metaphysis showing the direct connection between a CD31hi (green) Emcn− (red) α-SMA+ artery (white) and CD31hi Emcnhi type H capillaries. Dashed line, growth plate. c, Representative confocal tile scan of 2-week-old Efnb2GFP/+ (green) tibia stained with Emcn (red). Note GFP signal in arteries (arrows) and type H vessels in metaphysis and endosteum (arrowhead), while type L vessels lack Efnb2GFP/+ expression.
Extended Data Figure 2 Relationship between type H capillaries and arterioles.
a, Tile scan (left) and different representations (merged channels) of image data in inset. Shown is a 4-week-old tibial metaphysis immunostained for CD31 (green), Emcn (red) and α-SMA (white). CD31hi (green) Emcn− arteries (arrowheads) are directly connecting to type H ECs (arrows). ES, endosteum. b, Maximum intensity projections of 4-week-old Efnb2GFP/+ (GFP, green) tibial metaphysis and diaphysis immunostained for CD31 (white) and Emcn (red). Panels in centre and on the right different representations (merged channels) of image data. Only Emcn− arterial ECs (arrowheads) and Emcnhi type H ECs in metaphysis and endosteum (arrows) are positive for GFP, while diaphyseal type L ECs lack Efnb2GFP expression. c, Confocal images of Efnb2GFP/+ metaphysis showing GFP+ (green) Emcnhi (red) type H ECs (arrows) connecting to α-SMA+ (white) cell-covered, Emcn− artery (arrowhead). Nuclei, DAPI (blue). d, RT–qPCR analysis of Efnb2 and Sox17 expression (normalized to Actb) in sorted tibial CD31hi Emcnhi relative to CD31lo Emcnlo ECs. Data represent mean ± s.d. (n = 6; three independent experiments). P values, two-tailed unpaired t-test. UD, undetectable.
Extended Data Figure 3 Characterization of bone vessel populations.
a, b, Confocal (a) and tile scan confocal (b) images showing Emcn (red) and Sox17 (green) expression in 4-week-old tibia. Dashed line marks growth plate in the metaphysis. Sox17 is expressed by type H ECs (arrows) and arteries (arrowheads), whereas signal is absent in type L vessels of the diaphysis. c, Confocal images showing GFP (green), Emcn (red) and Sox17 (white) expression in the metaphysis region and diaphysis region of a Efnb2GFP/+ tibial section. Nuclei, DAPI (blue). Note Sox17 and GFP double-positive cells in type H capillaries (arrow) connecting to Efnb2GFP+ Emcn− artery (arrowhead). d, Representative confocal images from metaphysis (left) and diaphysis (right) regions of 4-week-old murine long bone showing Emcn (red), CD31 (green) and NRP1 (white) immunostaining. Nuclei, DAPI (blue). NRP1 marks type H capillaries (arrow) and arteries, while type L vessels lack detectable NRP1 expression. e, f, Representative confocal (e) and tile scan confocal (f) images showing Sca1 (red) and CD31 (green) immunostaining in metaphysis and diaphysis of 4-week-old tibia. Sca1 staining decorates CD31hi arteries (arrowheads) and type H ECs (arrows) but not type L ECs. Dashed line marks border of the growth plate. g, Maximum intensity projections of EdU (red) labelled 3-week-old tibia immunostained for CD31 (green) and α-SMA (white). Nuclei, DAPI (blue). Proliferating (EdU+) CD31hi type H ECs (arrow) were found near the growth plate (dashed line), whereas distal α-SMA+ cell-covered arteries (arrowheads) lack EC proliferation. h, Confocal images showing EdU labelling in 5-week-old Flk1–GFP long bone. Note EdU+ and GFP+ (arrows) in endothelial columns close to the growth plate. Straight, small calibre arterioles (arrowheads) lack proliferating ECs. i, Confocal images of 3-week-old Efnb2GFP/+ (green) tibia sections with α-SMA immunostaining (white). Ephrin-B2+ arteriolar ECs exhibit elongated nuclei (blue arrowheads), which are not typical for type H ECs. Overlap of GFP and EdU signals in type H capillaries (arrows) but not in arteries (blue arrowheads).
Extended Data Figure 4 Distribution of perivascular cells and HSCs.
a, PDGFRβ (green) cell localization in 4-week-old tibia. PDGFRβ+ cells surround arteries and Emcnhi (red) metaphyseal type H vessels but not diaphyseal sinusoidal vessels. b, Representative tile scan images of 4-week-old long bone immunostained for Emcn (red) and NG2 (green). Nuclei, DAPI (blue). Dashed lines mark growth plate and compact bone. Note abundance of NG2 signals around type H vessel columns (arrowhead) in metaphysis and arteries (arrow). c, Confocal images of 4-week-old NG2-DsRed (DsRed shown as green) transgenic long bone after CD31 immunostaining (red). Nuclei, DAPI (blue). Note abundance of DsRed+ cells around CD31hi (type H) capillaries (arrowhead). d, Sections of 3-week-old tibia immunostained for Emcn (red), NG2 (light blue) and PDGFRβ (green), as indicated. PDGFRβ+ cells were abundant around arteries (arrow) and type H vessels (arrowheads) in metaphysis. e, Graph showing CD150+ CD48− Lin− Sca1+ c-Kit+ HSC frequency in single-cell suspension. The metaphysis region from long bones was dissected and, subsequently, these bone fragments were crushed and subjected to collagenase digestion. Simultaneously, CD150+ CD48− Lin− Sca1+ c-Kit+ HSC frequency was also quantitated for BM flushed from diaphysis. Data represent mean ± s.e.m. (n = 5; three independent experiments). P values, two-tailed unpaired t-test. f, Maximum intensity projections showing CD31+ (red) arteries and type H vessels. CD150+ cells (green, arrowheads) in proximity of metaphyseal and endosteal endothelium as well as CD31+ arterioles (arrowheads). Top right, distribution of CD150+ and CD48+ cells in 4-week-old tibial metaphysis. Arrowheads in higher magnification of inset (bottom) mark CD150+ and CD48− HSCs. Dashed line marks growth plate. g, Perivascular localization of CD150+ (green) CD48− Lineage− (blue) HSCs (arrowhead) near Emcn+ (red) endothelium. h, Representative confocal image showing CD150+ cells (green) in the 4-week-old tibial metaphysis. Arrow marks rare CD150+ CD48− cell, which is also shown at high magnification in inset. Top panels show three-dimensional reconstruction of a thick section, while optical slices (mimicking thin sections) are shown at the bottom. Note that CD150+ CD48− cell (arrow) is only captured in one optical section and would therefore appear more isolated in thin tissue sections. i, Maximum intensity projections (top) showing CD150+ (green) cells in 4-week-old tibial metaphysis. Arrow marks rare CD150+ CD48− cell, arrowhead indicates nearby CD150+ megakaryocyte. Higher magnifications of inset show three-dimensional projection and thin optical slices, as indicated. General abundance of CD150+ cells appears strongly reduced in individual optical sections and the indicated CD150+ CD48− cell (arrow) is only captured in one slice. Notably, CD150+ cells were frequently found in clusters in thick cryosections (100 μm), but appeared scattered in thin optical slices, which reflects the reported widespread distribution throughout the BM.
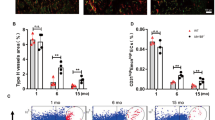
Extended Data Figure 5 Age-associated changes in bone vasculature.
a, Representative confocal images from the metaphysis of young (3-week-old) long bone and the corresponding region in aged (70-week-old) long bone after α-SMA (green) and Emcn (red) immunostaining. Note decline in α-SMA+ cell-covered arteries in aged samples. Growth plate and compact bone are indicated. b, Dot plots showing ephrin-B2+ ECs sorted by flow cytometry from a single-cell suspension isolated from long bone. Total ECs in the single-cell suspension were identified as CD45−/Ter119−/CD31+ cells. c, qPCR analysis of Kitl expression (normalized to Actb) by CD31hi Emcnhi type H ECs and arterial ECs relative to CD31lo Emcnlo ECs sorted from murine tibia. Arterial ECs were identified and sorted as Emcn− CD31+ ephrin-B2+ cells. Data represent mean ± s.d. (n = 6; in three independent experiments). P values, two-tailed unpaired t-test. Note significantly higher Kitl expression in type H and arterial ECs relative to type L ECs. d, Confocal images showing young (4-week-old) and aged (65-week-old) NG2-DsRed (red) long bone. Nuclei, DAPI (blue). e, Maximum intensity projections of sectioned metaphyseal regions from young (3-week-old) and aged (70-week-old) long bone immunostained for PDGFRβ (green) and Emcn (red). Nuclei, DAPI (blue). Dashed lines indicated the border to growth plate. Arrowheads indicate capillaries and arrow shows an artery. f, Representative confocal images showing NICD-Cre-induced GFP expression (green) in Emcn+ (red) type H capillaries (arrowheads) and Emcn− arteries (arrows) in 3-week-old tibia. Dashed lines mark growth plate or compact bone. g, Representative tile scan confocal images of 3-week-old tibia sections from NICD-Cre knock-in transgenic mice in the in the Rosa26-mT/mG reporter background. Vessels have been visualized by Emcn (red) immunostaining. Nuclei, DAPI (blue). Note high GFP expression in type H endothelium (arrows) and arteries (arrowheads). Dashed lines mark growth plate and endosteum; metaphysis and diaphysis are indicated. h, Contour plot showing intensities of CD31 immunostaining and GFP in single-cell suspension obtained from 3-week-old NICD-Cre Rosa26-mT/mG mice. ECs were demarcated as CD45− Ter119− CD31+. Note high GFP intensity in the CD31hi EC subset.
Extended Data Figure 6 Effect of endothelial Notch on the BM stroma.
a, b, Confocal images showing CD31 (green) and Emcn (red) immunostained tibia sections of NICDiO-EC (NICD) mutant and littermate control (a) or Fbxw7iΔEC mutant (b). Small, interconnected arterioles (arrowheads) were abundant in Notch gain-of-function mutants. Growth plate, metaphysis and diaphysis are indicated. c, Representative confocal images from NICDiO-EC mutant (NICD) and littermate control long bone after Emcn (red), CD31 (white) and PDGFRβ (green) immunostaining. Note strong accumulation of PDGFRβ+ cells in NICDiO-EC bone (arrowheads). d, Quantitative analysis of Sca1+ ECs and Sca1− ECs in Fbxw7iΔEC or NICDiOE-EC long bone relative to Cre-negative littermate controls. Data represent mean ± s.e.m. (n = 6). P values, two-tailed unpaired t-test. Total ECs were identified as CD45− Ter119− CD31+ cells, as represented in the FACS plots. Note significant increase of Sca1+ ECs in mutants, whereas Sca1− ECs remain comparable to control. e, Confocal tile scans of 4-week-old Fbxw7iΔEC, Dll4iΔEC and littermate control tibiae immunostained with Emcn (red) and α-SMA (green). Unless otherwise mentioned, data presented in the panels are based on three independent experiments.
Extended Data Figure 7 Endothelial Notch improves HSC niche function.
a, Representative dot plot showing the gating strategy used for defining the CD31− CD45− Ter119− Sca-1+ and PDGFRα+ MSCs. Graph in middle panel illustrates the flow cytometric quantification of CD31− CD45− Ter119− Sca-1+ PDGFRα+ MSCs in Fbxw7iΔEC and littermate control femurs. Note increase of Sca-1+ and PDGFRα+ MSCs in Fbxw7iΔEC mutants (n = 8). Graph on right shows quantification of fibroblast colony-forming units (CFU-F) from Fbxw7iΔEC and control femurs (n = 7). Data represent mean ± s.e.m. P values, two-tailed unpaired t-test. Both assays confirm a significant increase in MSC frequency. b, Transcripts associated with osteogenic (Sp7), chondrogenic (Acan) and adipogenic (Cfd) differentiation were quantified by RT–qPCR after 14 days of differentiation culture of mesenchymal stromal cells isolated from femur. Data represent mean ± s.d. (n = 6). P values, two-tailed unpaired t-test. No significant differences were seen for Fbxw7iΔEC and control cells in vitro. The differentiation potential of Fbxw7iΔEC cultured MSCs ex vivo was not altered. c, ELISA analysis of cellular SCF (lysate prepared from washed cells) in Fbxw7iΔEC (n = 8 and 7) and RbpjiΔEC (n = 7 and 9) long bone. Data represent mean ± s.d. P values, two-tailed unpaired t-test. d, Graphs showing ELISA analysis of extracellular SCF in Fbxw7iΔEC (n = 5) and RbpjiΔEC (n = 6 and 5) long bone. Data represent mean ± s.d. P values, two-tailed unpaired t-test. e, Increased chimaerism of Fbxw7iΔEC mutant (also shown as Fig. 2f, upper panel) relative to littermate control BM is shown after primary and secondary transplantation (at 4 months after transplantation). Donor-derived chimaerism was analysed by transplanting BM cells harvested from Fbxw7iΔEC mutant mice or littermate controls together with CD45.1 recipient-derived BM cells into lethally irradiated animals. For the secondary transplantation, 1 × 106 BM cells from CD45.1 mice that had previously undergone transplantation at 1:1 ratio at 7 months after transplantation were injected into lethally irradiated recipients. Data represent mean ± s.e.m. (n = 5 donors), P values, two-tailed unpaired t-test. f, Levels of donor-derived multi-lineage contribution were determined for Fbwx7iΔEC and control BM cells at 18 weeks after transplantation by flow cytometry. HSC-CRU frequency and statistical significance were determined using ELDA software (n = 3 mice per dilution). Note significant increase in the HSC frequency in the Fbxw7iΔEC mutant BM compared with littermate controls. g, Flow cytometric quantification of haematopoietic lineages in the Fbxw7iΔEC and control BM. Data represent mean ± s.e.m. (n = 14), P values, two-tailed unpaired t-test. Frequency of cells belonging to different haematopoietic lineages was not significantly altered in Fbxw7iΔEC mutants. h, Representative confocal images of EC-specific Notch2iΔEC or global Notch4 mutants (Notch4KO) and corresponding littermate control tibial bones after Emcn (red), PDGFRβ (blue) and Sca-1 (green) immunostaining. i, Flow cytometric quantitation of type H ECs in Notch2iΔEC and Notch4 knockout bones relative to controls. Data represent mean ± s.e.m. (n = 5 in two independent experiments), P values, two-tailed unpaired t-test. j. Flow cytometric quantitation of HSCs in Notch2iΔEC and Notch4 knockout BM relative to controls. Data represent mean ± s.e.m. (n = 5 in two independent experiments), P values, two-tailed unpaired t-test. k. Representative confocal images of 4-week-old wild-type tibia showing Notch3 (green) and Emcn (red) immunostaining. Note absence of Notch3 expression in bone ECs. Nuclei, DAPI (blue). Unless otherwise mentioned, data presented in figure panels are based on three independent experiments.
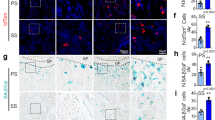
Extended Data Figure 8 HIF signalling in bone endothelium.
a, Maximum intensity projections of pimonidazole (green) stained young (8-week-old) and aged (45-week-old) tibia sections. Nuclei, DAPI (blue); CD31 immunostaining is shown in red. Dashed lines indicate the border of the growth plate. While pimonidazole staining was largely absent from 8-week-old metaphysis, hypoxic cells were readily detectable in the equivalent region in 45-week-old animals. b. Quantifications of Hif1a and Epas1 transcripts in sorted ECs from young (4-week-old) and old (60-week-old) bone. Data represent mean ± s.d. (n = 11). P values, two-tailed unpaired t-test. Endothelial expression of Hif1a transcripts was strongly reduced in aged animals, whereas expression of the related Epas1 (Hif2a) gene was significantly increased. c, Quantitative analysis of Cxcl12, Fgf1, Kitl, Tgfb3, Tgfb1 and Vegfa transcripts in dissected 60-week-old metaphysis relative to samples from young mice. Data represent mean ± s.d. (n = 5). P values, two-tailed unpaired t-test. d, Phospho-MAPK (phosphoERK1/2; green) and Emcn (red) immunostaining in young (4-week-old) and aged (50-week-old) metaphysis. Nuclei, DAPI (blue). Dashed lines mark growth plate. e, Representative tile scan confocal images obtained from control, Hif1aiΔEC and double-mutant Hif1aiΔEC VhliΔEC tibial sections. Immunostaining for Emcn (red) and Osterix (green) is shown. Nuclei, DAPI (blue). The decline of type H ECs and Osterix+ cells in Hif1aiΔEC bone was not recovered in Hif1aiΔEC VhliΔEC double mutants. f, Graph showing Fgf1, Pdgfa, Pdgfb, Tgfb1 and Tgfb3 transcript levels in sorted ECs from Hif1aiΔEC and Hif1aiΔEC VhliΔEC double-mutant bones normalized to littermate control. Data represent mean ± s.d. (n = 4–7). P values, two-tailed unpaired t-test. g, Representative confocal images showing immunostaining for CD31 (green) and Emcn (red) in Hif1aiΔEC mutant, Hif1aiΔEC VhliΔEC double-mutant and control bones. h, Flow cytometric analysis of Sca1+/− (n = 5) and ephrin-B2+/− ECs (n = 5 and 6) among total CD45− Ter119− CD31+ ECs in Hif1aiΔEC long bones relative to Cre-negative littermates. Data represent mean ± s.e.m. P values, two-tailed unpaired t-test. i, Representative confocal images of Hif1aiΔEC or control tibial metaphysis after Emcn (red) and PDGFRβ (green) immunostaining. Nuclei, DAPI (blue). Dashed lines mark the growth plate. Note decline in Emcnhi ECs and PDGFRβ+ perivascular cells in Hif1aiΔEC mutant. j, Analysis of donor-derived cells indicating LTR-HSC contribution, as determined 7 months after transplantation by flow cytometry. BM cells harvested from Hif1aiΔEC mutant mice or littermate controls were transplanted together with recipient CD45.1 recipient-derived BM cells into lethally irradiated recipient mice. Data represent mean ± s.e.m. (n = 6 donors). P values, two-tailed unpaired t-test. k, Quantitative analysis of Sca1+/− ECs (n = 6 and 5) and ephrin-B2+/− ECs (n = 5 and 4) among total CD45− Ter119− CD31+ ECs in VhliΔEC long bone relative to Cre-negative littermates. Data represent mean ± s.e.m. (n = 4–6). P values, two-tailed unpaired t-test. l, Representative confocal images of VhliΔEC and control tibial metaphysis with Emcn (red) and PDGFRβ (green) immunostaining. Nuclei, DAPI (blue). Dashed lines mark growth plate. Unless otherwise mentioned, data presented in figure panels are based on three independent experiments.
Extended Data Figure 9 Relation between Notch and HIF in bone ECs.
a, Metaphysis region of 2-week-old tibia after CD31 (red) and HIF-1α (green) immunostaining. Dashed line marks the growth plate; arrows indicate type H endothelium. Note the presence of HIF-1α in type H ECs (arrows) but the absence of signal in CD31+ arteries (arrowheads in right panel). b, Confocal images showing CD31 (green) and Emcn (red) immunostaining of VhliΔEC mutant and littermate control tibia sections. Dashed lines mark growth plate. Note increase in type H capillaries. c, Tile scan confocal images showing α-SMA (green) and Emcn (red) immunostained tibia. Similar amounts of α-SMA+ cell-covered arteries were visible in VhliΔEC and control samples. d, e, ELISA analysis of cellular (cell lysates, d) and secreted SCF levels (cell culture supernatant, e) in cell lysates of cultured BM-derived ECs and PDGFRβ+ perivascular cells (PVCs; n = 5 replicates) after treatment with vehicle control or DFM. Data represent mean ± s.d. P values, one-way ANOVA with Tukey’s multiple comparison post-hoc test. f, g, Frequency (percentage) of type H ECs (f, n = 4) and PDGFRβ+ PVCs (g, n = 5 mutants and 6 controls) among total BM cells in Hif1aiΔEC NICDiOEC, RbpjiΔEC VhliΔEC and NICDiOEC VhliΔEC double mutants relative to Cre-negative controls. Data represent mean ± s.e.m. P values, one-way ANOVA with Tukey’s multiple comparison post-hoc test. The combination of enhanced Notch and HIF activity in NICDiOE-EC VhliΔEC double mutants failed to induce a bigger expansion of type H ECs. h, ELISA analysis of the cellular SCF levels in lysates of femur cells from Hif1aiΔEC NICDiOEC, RbpjiΔEC VhliΔEC and NICDiOEC VhliΔEC double mutants relative to Cre-negative controls. Data represent mean ± s.e.m. (n = 4 or 5 mutants and 5 controls). P values, one-way ANOVA with Tukey’s multiple comparison post-hoc test. i, HSC frequency (%) in Hif1aiΔEC NICDiOEC, RbpjiΔEC VhliΔEC and NICDiOEC VhliΔEC double mutants relative to Cre-negative controls. Data represent mean ± s.e.m. (n = 4 or 5 mutants and 4 controls). P values, one-way ANOVA with Tukey’s multiple comparison post-hoc test. Unless otherwise mentioned, data presented in figure panels are based on three independent experiments.
Extended Data Figure 10 Properties of vascular niches and HSCs in aged mice.
a, qPCR analysis of Pdgfrb and Cspg4 expression (normalized to Actb) in long bone of Fbxw7iΔEC mutants relative to littermate controls. Data represent mean ± s.d. (n = 4 left panel; n = 6 right panel). P values, two-tailed unpaired t-test. b, Analysis of LTR-HSC contribution of BM cells from aged Fbxw7iΔEC and control donors, as determined by flow cytometry at 16 weeks after competitive transplantation together with young CD45.1 BM cells (from 12- to 14-week-old mice) into lethally irradiated recipients. Data represent mean ± s.e.m. (n = 6 donors). c, Levels of donor-derived multi-lineage contribution of aged Fbwx7iΔEC and age-matched control BM cells as determined 18 weeks after transplantation by flow cytometry analysis. HSC-CRU frequency and statistical significance were determined using ELDA software (n = 3 mice per dilution). d, Donor-derived lymphoid and myeloid contributions of aged Fbxw7iΔEC and control BM cells determined 18 weeks after transplantation by flow cytometry analysis with B220 and CD11b antibodies. Data represent mean ± s.d. (n = 24). P values, two-tailed unpaired t-test. e, Representative images and quantification of γ-H2AX foci in Lineage−/cKit+/Sca-1+ haematopoietic stem and progenitor cells (HSPCs) sorted from Fbwx7iΔEC mice and littermate controls (110 HSPCs were scored for each group). Note persistence of γ-H2AX foci in the aged Fbxw7iΔEC HSPCs. Unless otherwise mentioned, data presented in figure panels are based on three independent experiments.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1, a list of antibodies. (PDF 141 kb)
Rights and permissions
About this article
Cite this article
Kusumbe, A., Ramasamy, S., Itkin, T. et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384 (2016). https://doi.org/10.1038/nature17638
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17638
This article is cited by
-
MarShie: a clearing protocol for 3D analysis of single cells throughout the bone marrow at subcellular resolution
Nature Communications (2024)
-
The roles of bone remodeling in normal hematopoiesis and age-related hematological malignancies
Bone Research (2023)
-
The roles of tertiary lymphoid structures in chronic diseases
Nature Reviews Nephrology (2023)
-
Spatial and single-cell profiling of the metabolome, transcriptome and epigenome of the aging mouse liver
Nature Aging (2023)
-
Spatial heterogeneity of bone marrow endothelial cells unveils a distinct subtype in the epiphysis
Nature Cell Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.