Abstract
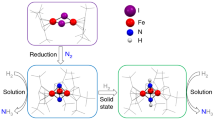
Although the oxidation of water is efficiently catalysed by the oxygen-evolving complex in photosystem II (refs 1 and 2), it remains one of the main bottlenecks when aiming for synthetic chemical fuel production powered by sunlight or electricity. Consequently, the development of active and stable water oxidation catalysts is crucial, with heterogeneous systems3,4 considered more suitable for practical use and their homogeneous counterparts more suitable for targeted, molecular-level design guided by mechanistic understanding5,6,7,8,9,10,11,12,13,14,15,16,17,18,19. Research into the mechanism of water oxidation has resulted in a range of synthetic molecular catalysts, yet there remains much interest in systems that use abundant, inexpensive and environmentally benign metals such as iron (the most abundant transition metal in the Earth’s crust and found in natural20,21 and synthetic22 oxidation catalysts). Water oxidation catalysts based on mononuclear iron complexes have been explored9,12,16,18, but they often deactivate rapidly and exhibit relatively low activities. Here we report a pentanuclear iron complex that efficiently and robustly catalyses water oxidation with a turnover frequency of 1,900 per second, which is about three orders of magnitude larger than that of other iron-based catalysts. Electrochemical analysis confirms the redox flexibility of the system, characterized by six different oxidation states between FeII5 and FeIII5; the FeIII5 state is active for oxidizing water. Quantum chemistry calculations indicate that the presence of adjacent active sites facilitates O–O bond formation with a reaction barrier of less than ten kilocalories per mole. Although the need for a high overpotential and the inability to operate in water-rich solutions limit the practicality of the present system, our findings clearly indicate that efficient water oxidation catalysts based on iron complexes can be created by ensuring that the system has redox flexibility and contains adjacent water-activation sites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Umena, Y., Kawakami, K., Shen, J.-R. & Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60 (2011)
Dismukes, G. C. et al. Development of bioinspired Mn4O4 – cubane water oxidation catalysts: lessons from photosynthesis. Acc. Chem. Res. 42, 1935–1943 (2009)
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008)
Hocking, R. K. et al. Water-oxidation catalysis by manganese in a geochemical-like cycle. Nature Chem. 3, 461–466 (2011)
Gersten, S. W., Samuels, G. J. & Meyer, T. J. Catalytic oxidation of water by an oxo-bridged ruthenium dimer. J. Am. Chem. Soc. 104, 4029–4030 (1982)
Wada, T., Tsuge, K. & Tanaka, K. Electrochemical oxidation of water to dioxygen catalyzed by the oxidized form of the bis(ruthenium-hydroxo)complex in H2O. Angew. Chem. Int. Edn 39, 1479–1482 (2000)
Zong, R. & Thummel, R. P. A new family of Ru complexes for water oxidation. J. Am. Chem. Soc. 127, 12802–12803 (2005)
Concepcion, J. J., Jurss, J. W., Templeton, J. L. & Meyer, T. J. One site is enough. Catalytic water oxidation by [Ru(tpy)(bpm)(OH2)]2+ and [Ru(tpy)(bpz)(OH2)]2+. J. Am. Chem. Soc. 130, 16462–16463 (2008)
Ellis, W. C., McDaniel, N. D., Bernhard, S. & Collins, T. J. Fast water oxidation using iron. J. Am. Chem. Soc. 132, 10990–10991 (2010)
Yin, Q. et al. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 328, 342–345 (2010)
Yoshida, M., Masaoka, S., Abe, J. & Sakai, K. Catalysis of mononuclear aquaruthenium complexes in oxygen evolution from water: a new radical coupling path using hydroxocerium(IV) species. Chem. Asian J. 5, 2369–2378 (2010)
Fillol, J. L. et al. Efficient water oxidation catalysts based on readily available iron coordination complexes. Nature Chem. 3, 807–813 (2011)
Duan, L. et al. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nature Chem. 4, 418–423 (2012)
Barnett, S. M., Goldberg, K. I. & Mayer, J. M. A soluble copper-bipyridine water-oxidation electrocatalyst. Nature Chem. 4, 498–502 (2012)
Vigara, L. et al. Experimental and quantum chemical characterization of the water oxidation cycle catalysed by [RuII(damp)(bpy)(H2O)]2+. Chem. Sci. 3, 2576–2586 (2012)
Hong, D. et al. Water oxidation catalysis with nonheme iron complexes under acidic and basic conditions: homogeneous or heterogeneous? Inorg. Chem. 52, 9522–9531 (2013)
Wang, D. & Groves, J. T. Efficient water oxidation catalyzed by homogeneous cationic cobalt porphyrins with critical roles for the buffer base. Proc. Natl Acad. Sci. USA 110, 15579–15584 (2013)
Coggins, M. K., Zhang, M.-T., Vannucci, A. K., Dares, C. J. & Meyer, T. J. Electrocatalytic water oxidation by a monomeric amidate-ligated Fe(III)–aqua complex. J. Am. Chem. Soc. 136, 5531–5534 (2014)
Matheu, R. et al. Intramolecular proton transfer boosts water oxidation catalyzed by a Ru complex. J. Am. Chem. Soc. 137, 10786–10795 (2015)
Meunier, B., de Visser, S. P. & Shaik, S. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem. Rev. 104, 3947–3980 (2004)
Tinberg, C. E. & Lippard, S. J. Dioxygen activation in soluble methane monooxygenase. Acc. Chem. Res. 44, 280–288 (2011)
Costas, M., Chen, K. & Que, L. Jr Biomimetic nonheme iron catalysts for alkane hydroxylation. Coord. Chem. Rev. 200–202, 517–544 (2000)
Einsle, O. et al. Nitrogenase MoFe-protein at 1.16 Å resolution: a central ligand in the FeMo-cofactor. Science 297, 1696–1700 (2002)
Kodera, M. et al. Reversible O-O bond scission of peroxodiiron(III) to high-spin oxodiiron(IV) in dioxygen activation of a diiron center with a bis-tpa dinucleating ligand as a soluble methane monooxygenase model. J. Am. Chem. Soc. 134, 13236–13239 (2012)
Kundu, S. et al. O-O bond formation mediated by a hexanuclear iron complex supported on a stannoxane core. Chem. Eur. J. 18, 2787–2791 (2012)
Yoneda, K. et al. An [FeII3O]4+ core wrapped by two [FeIIL3]− units. Angew. Chem. Int. Edn 45, 5459–5461 (2006)
Helm, M. L., Stewart, M. P., Bullock, R. M., DuBois, M. R. & DuBois, D. L. A synthetic nickel electrocatalyst with a turnover frequency above 100,000 s−1 for H2 production. Science 333, 863–866 (2011)
McCrory, C. C. L., Uyeda, C. & Peters, J. C. Electrocatalytic hydrogen evolution in acidic water with molecular cobalt tetraazamacrocycles. J. Am. Chem. Soc. 134, 3164–3170 (2012)
Marinescu, S. C., Winkler, J. R. & Gray, H. B. Molecular mechanisms of cobalt-catalyzed hydrogen evolution. Proc. Natl Acad. Sci. USA 109, 15127–15131 (2012)
Costentin, C., Drouet, S., Robert, M. & Savéant, J.-M. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst. Science 338, 90–94 (2012)
Acknowledgements
This study was supported by MEXT/JSPS Grants-in-Aid as follows: for Young Scientists A (no. 25708011 (S.M.) and no. 15H05480 (M.K.)), for Challenging Exploratory Research (no. 26620160 (S.M.)), for Scientific Research on Innovative Areas (''AnApple'' (no. 15H00889 (S.M.) and no. 25107526 (S.M.)), for Photosynergetics (no. 15H01097 (T.Y.)), for Soft Molecular Systems (no. 26104538 (Y.K.)), for a JSPS Fellowship (no. 254037 (M.O.)), for Scientific Research B (no. 25288013 (T.Y.)), and for Scientific Research C (no. 25410030 (Y.K.)). This work was also supported by JST ACT-C (M.K.), JST PRESTO (Y.K. and S.M.), the NINS Program for Cross-Disciplinary Study (S.M.) and the Morino Foundation for Molecular Science (Y.K.). The computations were performed using the Research Center for Computational Science, Okazaki, Japan. We also thank S. Furukawa for discussions.
Author information
Authors and Affiliations
Contributions
M.O., M.K. and S.M. conceived the project. M.O., M.K., M.Y., K.Y., S.K. and S.M. designed the experiments. M.O. and R.K. performed all synthesis and characterisation experiments. Y.K. and T.Y. performed the DFT calculations. S.H. measured the Mössbauer spectra. V.K.K.P. performed the isolation of reaction intermediates. M.O., M.K. and S.M. analysed the data and co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
The X-ray crystal structure of 1(BF4)3 is deposited in the Cambridge Crystallographic Data Centre (CCDC 996195).
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Figures 1-30, Supplementary Tables 1-13 and Supplementary References– see contents for details. (PDF 4699 kb)
Supplementary Data
This file contains crystallographic information files (ZIP 149 kb)
Video 1: Water attack reaction to the S4 state (LS state, side view)
This video shows the calculated water insertion reaction pathway to the four-electron oxidised (S4) state of the pentanuclear iron complex (S4 + H2O →A). The MS parameter (total spin quantum number) of 18 was used in this calculation. For the details of the calculation, see also Supplementary Information (P.S28-33) (MP4 2085 kb)
Video 2: Water attack reaction to the S4 state (LS state, top view)
This video shows the calculated water insertion reaction pathway to the four-electron oxidised (S4) state of the pentanuclear iron complex (S4 + H2O →A) along with the profile of relative energies. The MS parameter (total spin quantum number) of 18 was used in this calculation. For the details of the calculation, see also Supplementary Information (P.S28-33) (MP4 1593 kb)
Video 3: Water attack reaction to the S4 state (HS state, side view)
This video shows the calculated water insertion reaction pathway to the four-electron oxidised (S4) state of the pentanuclear iron complex (S4 + H2O →A). The MS parameter (total spin quantum number) of 26 was used in this calculation. For the details of the calculation, see also Supplementary Information (P.S28-33) (MP4 2060 kb)
Video 4: Water attack reaction to the S4 state (HS state, top view)
This video shows the calculated water insertion reaction pathway to the four-electron oxidised (S4) state of the pentanuclear iron complex (S4 + H2O →A) along with the profile of relative energies. The MS parameter (total spin quantum number) of 26 was used in this calculation. For the details of the calculation, see also Supplementary Information (P.S28-33) (MP4 1479 kb)
Rights and permissions
About this article
Cite this article
Okamura, M., Kondo, M., Kuga, R. et al. A pentanuclear iron catalyst designed for water oxidation. Nature 530, 465–468 (2016). https://doi.org/10.1038/nature16529
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature16529
This article is cited by
-
Theoretical elucidation of the structure, bonding, and reactivity of the CaMn4Ox clusters in the whole Kok cycle for water oxidation embedded in the oxygen evolving center of photosystem II. New molecular and quantum insights into the mechanism of the O–O bond formation
Photosynthesis Research (2023)
-
Solar utilization beyond photosynthesis
Nature Reviews Chemistry (2022)
-
Nickel dual-atom sites for electrochemical carbon dioxide reduction
Nature Synthesis (2022)
-
Redox-active ligands in artificial photosynthesis: a review
Environmental Chemistry Letters (2022)
-
Oxygen Evolution Reaction in Energy Conversion and Storage: Design Strategies Under and Beyond the Energy Scaling Relationship
Nano-Micro Letters (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.