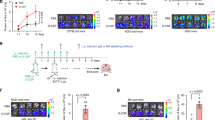
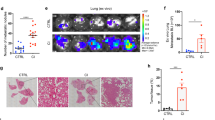
Abstract
Despite progress in the development of drugs that efficiently target cancer cells, treatments for metastatic tumours are often ineffective. The now well-established dependency of cancer cells on their microenvironment1 suggests that targeting the non-cancer-cell component of the tumour might form a basis for the development of novel therapeutic approaches. However, the as-yet poorly characterized contribution of host responses during tumour growth and metastatic progression represents a limitation to exploiting this approach. Here we identify neutrophils as the main component and driver of metastatic establishment within the (pre-)metastatic lung microenvironment in mouse breast cancer models. Neutrophils have a fundamental role in inflammatory responses and their contribution to tumorigenesis is still controversial2,3,4. Using various strategies to block neutrophil recruitment to the pre-metastatic site, we demonstrate that neutrophils specifically support metastatic initiation. Importantly, we find that neutrophil-derived leukotrienes aid the colonization of distant tissues by selectively expanding the sub-pool of cancer cells that retain high tumorigenic potential. Genetic or pharmacological inhibition of the leukotriene-generating enzyme arachidonate 5-lipoxygenase (Alox5) abrogates neutrophil pro-metastatic activity and consequently reduces metastasis. Our results reveal the efficacy of using targeted therapy against a specific tumour microenvironment component and indicate that neutrophil Alox5 inhibition may limit metastatic progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nature Med. 19, 1423–1437 (2013)
Bald, T. et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 507, 109–113 (2014)
Galdiero, M. R. et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology 218, 1402–1410 (2013)
Finisguerra, V. et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature 522, 349–353 (2015)
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature Cell Biol. 8, 1369–1375 (2006)
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009)
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005)
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2012)
Calon, A. et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22, 571–584 (2012)
Oskarsson, T., Batlle, E. & Massagué, J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 14, 306–321 (2014)
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015)
Luu, N. T., Rainger, G. E., Buckley, C. D. & Nash, G. B. CD31 regulates direction and rate of neutrophil migration over and under endothelial cells. J. Vasc. Res. 40, 467–479 (2003)
Condamine, T., Ramachandran, I., Youn, J. I. & Gabrilovich, D. I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 66, 97–110 (2015)
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011)
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013)
Korkaya, H., Liu, S. & Wicha, M. S. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J. Clin. Invest. 121, 3804–3809 (2011)
Tsuyada, A. et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 72, 2768–2779 (2012)
Yan, H. H. et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 70, 6139–6149 (2010)
Snoussi, K., Strosberg, A. D., Bouaouina, N., Ben Ahmed, S. & Chouchane, L. Genetic variation in pro-inflammatory cytokines (interleukin-1β, interleukin-1α and interleukin-6) associated with the aggressive forms, survival, and relapse prediction of breast carcinoma. Eur. Cytokine Netw. 16, 253–260 (2005)
Wang, D. & Dubois, R. N. Eicosanoids and cancer. Nature Rev. Cancer 10, 181–193 (2010)
Cho, N. K., Joo, Y. C., Wei, J. D., Park, J. I. & Kim, J. H. BLT2 is a pro-tumorigenic mediator during cancer progression and a therapeutic target for anti-cancer drug development. Am. J. Cancer Res. 3, 347–355 (2013)
Kanaoka, Y. & Boyce, J. A. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J. Immunol. 173, 1503–1510 (2004)
Hiraga, T., Ito, S. & Nakamura, H. Side population in MDA-MB-231 human breast cancer cells exhibits cancer stem cell-like properties without higher bone-metastatic potential. Oncol. Rep. 25, 289–296(2011)
Sheridan, C. et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 8, R59 (2006)
Lo, P. K. et al. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFβ signaling. Oncogene 31, 2614–2626 (2012)
Park, M. K. et al. Novel involvement of leukotriene B4 receptor 2 through ERK activation by PP2A down-regulation in leukotriene B4-induced keratin phosphorylation and reorganization of pancreatic cancer cells. Biochim. Biophys. Acta 1823, 2120–2129 (2012)
Wenzel, S. E. & Kamada, A. K. Zileuton: the first 5-lipoxygenase inhibitor for the treatment of asthma. Ann. Pharmacother. 30, 858–864 (1996)
Donskov, F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin. Cancer Biol. 23, 200–207 (2013)
Han, Y. et al. Prognostic value of chemotherapy-induced neutropenia in early-stage breast cancer. Breast Cancer Res. Treat. 131, 483–490 (2012)
Guy, C. T., Cardiff, R. D. & Muller, W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell. Biol. 12, 954–961 (1992)
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 (1997)
Lieschke, G. J. et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84, 1737–1746 (1994)
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992)
Cao, Y. A. et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc. Natl Acad. Sci. USA 101, 221–226 (2004)
Ivanova, A. et al. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis 43, 129–135 (2005)
Tkalcevic, J. et al. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity 12, 201–210 (2000)
Chen, X. S., Sheller, J. R., Johnson, E. N. & Funk, C. D. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature 372, 179–182 (1994)
Daley, J. M., Thomay, A. A., Connolly, M. D., Reichner, J. S. & Albina, J. E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70 (2008)
Bao, Y. & Cao, X. Revisiting the protective and pathogenic roles of neutrophils: Ly-6G is key! Eur. J. Immunol. 41, 2535–2538 (2011)
Acknowledgements
We thank C. Reis e Sousa, E. Sahai, P. Scaffidi and J. Huelsken for scientific discussions, critical reading of the manuscript and sharing cell lines and mouse strains. We also thank members of the tumour-stroma interactions in cancer development (TSI) laboratory of The Crick Institute for scientific discussions, critical reading of the manuscript and practical support. We thank L. Jones for help in analysing the human breast cancer samples. We are grateful to R. Moore, E. Nye, B. Spencer-Dene and J. Bee for technical support with mice and mouse tissue. We also thank the Flow Cytometry Unit, the Bioinformatics & Biostatistics Unit and the In vivo Imaging Facility for technical assistance. We are grateful to Cancer Research UK for the funding that has allowed this work.
Author information
Authors and Affiliations
Contributions
S.K.W. organised and performed all experiments, helped design experiments, interpreted data and helped with manuscript preparation. I.M. conceived and supervised the study, designed experiments, interpreted the data, assisted with some aspects of the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Mammary tumour-bearing MMTV-PyMT+ mice show specifically neutrophilia in the metastatic lung.
a–c, Flow cytometric quantification of CD11b+Ly6G+ neutrophils in the bone marrow (n = 6 (wild type), n = 5 (MMTV-PyMT+)) (a), liver (n = 4 (wild type), n = 5 (MMTV-PyMT+)) (b) and spleen (n = 6 (wild type), n = 5 (MMTV-PyMT+)) (c) of wild-type (WT) and tumour-bearing MMTV-PyMT+ mice. d, Quantification of neutrophils in the tumour and metastatic lung of MMTV-PyMT+ mice (n = 2 per group), pre-metastatic lung neutrophil levels depicted in Fig. 1a are shown for comparison in dashed lines. Met., metastatic. e–l, Flow cytometric quantification of immune cell frequencies in wild-type and metastatic lungs of MMTV-PyMT+ mice (n = 4 (wild type), n = 7 (metastatic) if not otherwise indicated) including CD45+ total immune cells (e), total CD11b+F4/80+ macrophages (f) (n = 4 (wild type), n = 4 (metastatic)), the CD11blow F4/80high alveolar macrophage subpopulation (n = 4 (wild type), n = 4 (metastatic)) (g), the CD11bhigh F4/80low interstitial macrophage subpopulation (n = 4/WT, n = 4/Met.) (h), CD45+CD11c+ dendritic cells (i), CD45+CD49b+ NK cells (j), CD45+CD19+ B lymphocytes (k) and CD45+CD3+ T lymphocytes (l). Statistical analysis by two-sided t-test. Data are represented as mean ± s.e.m. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Figure 2 Analysis of MMTV-PyMT+Gcsf−/− mice, G-CSF-deficient MMTV-PyMT cancer cells and MMTV-PyMT+Ela2-Cre-DTA+ mice.
a, Representative flow cytometric analysis of CD11b+Ly6G+ neutrophils in the lung of wild-type and tumour-bearing MMTV-PyMT+Gcsf+/+and MMTV-PyMT+Gcsf−/− mice. b, Primary mammary tumour burden of MMTV-PyMT+Gcsf+/+ (n = 13) or MMTV-PyMT+Gcsf−/− (n = 24) mice. c, Flow cytometric quantification of frequencies of total CD11b+F4/80+ macrophages (left), the CD11blowF4/80high alveolar macrophage subpopulation (middle) and the CD11bhighF4/80low interstitial macrophage subpopulation (right) in the lung of tumour-bearing MMTV-PyMT+Gcsf+/+ (n = 4) and MMTV-PyMT+Gcsf−/− (n = 7) mice. d, MMTV-PyMT+Gcsf−/− primary cancer cells were freshly isolated and grafted onto two mammary glands of Rag1-null mice (106 cells per injection) and analysed 5 weeks thereafter. CD11b+Ly6G+ neutrophil presence in the lung was assessed by flow cytometry (left), primary tumour burden was assessed by weighing (middle) and spontaneous lung metastasis incidence was assessed by quantification of visible surface lung metastases relative to tumour load (right) (n = 3 (Gcsf+/+), n = 4 (Gcsf−/−)). e–g, Analysis of tumour-bearing MMTV-PyMT+ control and MMTV-PyMT+Ela2-Cre-DTA+ mice. Representative flow cytometric analysis of CD11b+Ly6G+ neutrophils in the lung (e). Lung neutrophil quantification (n = 8 (wild type), n = 7 (PyMT+control), n = 5 (PyMT+Ela2-Cre-DTA+)) (f, left) and primary mammary tumour burden (n = 14 (PyMT+control), n = 6 (PyMT+Ela2-Cre-DTA+)) (f, right) with representative H&E-stained histological lung sections (g). Scale bar, 500 μm. h, Flow cytometric quantification of frequencies of total CD11b+F4/80+ macrophages (left), the CD11blowF4/80high alveolar macrophage subpopulation (middle) and the CD11bhighF4/80low interstitial macrophage subpopulation (right) in the lung of tumour-bearing MMTV-PyMT+ control (n = 7) and MMTV-PyMT+Ela2-Cre-DTA+ (n = 5) mice. i, Frequencies of bone marrow (top) and blood (bottom) CD11b+Ly6G+ neutrophils (left; blood n = 3 (wild type), n = 6 (PyMT+control)), CD11b+F4/80+ macrophages (middle; blood n = 3 (wild type), n = 6 (PyMT+control)) and CD11b+CD115+ monocytes (right; blood n = 3 (wild type), n = 5 (PyMT+control)) in wild-type, MMTV-PyMT+ control and MMTV-PyMT+Ela2-Cre-DTA+ mice analysed by flow cytometry (n = 4 (wild type), n = 6 (PyMT+control), n = 2 (PyMT+Ela2-Cre-DTA+) if not otherwise indicated). j, Exclusion of immune responses against DTA expression in the bone marrow by analysis of NK-cell (left) and cytotoxic T-cell (right) activation. Flow cytometric quantification of activated CD69+ among total CD45+CD49b+ NK cells as well as activated CD44+ or CD69+ among total CD45+CD3+CD8+ cytotoxic T cells in the bone marrow of wild-type (n = 4), MMTV-PyMT+ control (n = 6) and MMTV-PyMT+Ela2-Cre-DTA+ (n = 2) mice. Statistical analysis by two-sided t-test. Data are represented as mean ± s.e.m. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Figure 3 Comparison of wild-type lung neutrophils with tumour-induced, pre-metastatic lung neutrophils.
a, Representation of timing and dynamics of neutrophil and cancer cell infiltration into the lung of mice grafted with two mammary tumours by orthotopic injection of 106 MMTV-PyMT tumour cells. b, Flow cytometric analysis for cell size (forward scatter (FSC)), granularity (side scatter (SSC)) and expression of surface markers CXCR2, CD31, MHC-I, MHC-II, ICAM1 and Fas (n is indicated) as well as mRNA expression analysis of Tnfa, arginase 1, Vegfa, Ccl2, Ccl3, iNOS (also known as Nos2) and Ccl5 by quantitative polymerase chain reaction (PCR) of CD11b+Ly6G+ wild-type (WT) or pre-metastatic (Pre-met.) lung neutrophils 3 weeks after primary tumour graft (n = 3 (pre-metastatic compared with one normal lung reference)). Statistical analysis by two-sided t-test (flow cytometry) and one-sample t-test (mRNA). Data are represented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Figure 4 Immune cell frequencies and activation in the pre-metastatic lung of MMTV-PyMT tumour-bearing mice is not dependent on neutrophil presence (part 1).
a, Representation of timing and dynamics of neutrophil and cancer cell infiltration into the lung of mice grafted with two mammary tumours by orthotopic injection of 106 MMTV-PyMT tumour cells. b–o, Flow cytometric quantification and representative analysis of the following immune cell types in wild-type (WT) or pre-metastatic (Pre-met.) lungs treated daily with either control IgG or anti-Ly6G (1A8) neutrophil-blocking antibody from tumour onset onwards (n = 4 per group if not otherwise indicated): b, f, total CD45+ immune cells (n = 12 per group); c, g, CD11b+Ly6G+ neutrophils (n = 8 per group); d, g, CD11b+SiglecF+ eosinophils; e, g, CD11blowF4/80high alveolar macrophages and CD11bhighF4/80low interstitial macrophages; h, j, CD45+CD11c+ dendritic cells; i, k, MHC-II+CD86+ activated dendritic cells; l, n, CD45+CD19+ B cells; and m, o, MHC-II+CD86+ activated B cells. Statistical analysis by two-sided t-test. Data are represented as mean ± s.e.m. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Figure 5 Immune cell frequencies and activation in the pre-metastatic lung of MMTV-PyMT tumour-bearing mice is not dependent on neutrophil presence (part 2).
a–i, Flow cytometric quantification and representative analysis of the following immune cell types in wild-type (WT) or pre-metastatic (Pre-met.) lungs treated daily with either control IgG or anti-Ly6G (1A8) neutrophil-blocking antibody from tumour onset onwards (n = 4 per group if not otherwise indicated): a, c, CD45+CD49b+ NK cells; b, c, CD69+ activated NK cells; d, e, CD45+CD3+CD8+ cytotoxic T cells (n = 8 per group); f, g, CD44+ or CD69+ activated T cells; and h, i, the ratio of CD4+CD25+Foxp3+ regulatory T cells per activated T cell. Statistical analysis by two-sided t-test. Data are represented as mean ± s.e.m. NS, not significant.
Extended Data Figure 6 Neutrophil isolation from the lung of MMTV-PyMT+ mice and effect of neutrophil-derived factors on tumour formation potential.
a, Representative flow cytometric analysis of neutrophil purity after isolation from the pre-metastatic lung compared to total lung tissue. Only neutrophil purity of ≥90% was used for further experiments. b, Neutrophil viability was assessed by flow cytometry for propidium iodide (PI) negativity after isolation (n = 10). c, d, MMTV-PyMT cells grown in control or LuN medium for 3 days in adherent conditions were plated in non-attachment conditions followed by sphere quantification at day 10 post-seeding (technical replicate n = 17 (control), n = 21 (LuN) of biological triplicates) (c) or 104 cells grafted onto the mammary gland of Rag1-null mice for analysis of tumour formation potential (d). Tumour burden was determined by weighing about 3 weeks after (n = 12 per group), complementary to Fig. 2d. e–h, Flow cytometric quantification of frequencies of total present GFP-labelled MMTV-PyMT cells (e, g) and frequencies of CD24+CD90+ MICs among total GFP-labelled MMTV-PyMT cells (f, h) in the lung of Rag1-null mice 3 days after intravenous injection of 5 × 105 total GFP-labelled MMTV-PyMT cells followed by either three intravenous injections with control or LuN medium (n = 6 (PyMT+control), n = 8 (PyMT+LuN)) (e, f) or by one intravenous injection with 25 × 106 neutrophils freshly isolated from a pre-metastatic lung (n = 7 (PyMT+control), n = 8 (PyMT+neutrophils) (g, h). f, h, Two independent experiments are shown to complement Fig. 2h, i. Exp, experiment. i–k, Experimental setup (i): Rag1-null mice were intravenously injected with 1–10 × 105 (j) or 0.5 × 106 total GFP-labelled MMTV-PyMT cells (k) followed by either 3–5 intravenous injections with 200 μl control or LuN medium (j) or by 3 intravenous injections with 25 × 106 neutrophils (k) freshly isolated from a pre-metastatic lung. Quantification of experimental metastatic incidence by determination of bioluminescence intensity (n = 7 (control), n = 9 (LuN)) (j) or flow cytometric analysis of GFP+ cancer cells in the lung (n = 5 (control), n = 4 (neutrophil)) (k) is shown. Statistical analysis by two-sided t-test (c, j, k) and two-way ANOVA (d–h). Data are represented as mean ± s.e.m. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Figure 7 LTRs are expressed on mouse and human breast cancer cells and enriched on metastasis-initiating and highly tumorigenic cancer cell sub-pools.
a, Sphere formation potential of MMTV-PyMT cells under presence of LTB4 or LTC/D/E4 (technical replicate n = 8 per group of biological triplicates). b, c, Three-day LTB4 and LTC/D/E4-treated MMTV-PyMT cells in adherent culture were analysed for primary tumour initiation potential by orthotopic transplantation of 104 cells in Rag1-null mice (n = 14 per group) (b). Exp, experiment; TC, tumour cell isolation. Representative image of tumours is shown (c). d, e, Flow cytometric analysis of primary MMTV-PyMT cancer cells, the mouse mammary cancer cell line 4T1 and the human breast cancer cell line MDA-MB-231 for expression of BLT1 or BLT2 (d) as well as CysLT1 or CysLT2 (e). f, Representative flow cytometric analysis of BLT2+ and CysLT2+ cells among MMTV-PyMT non-MICs and MICs. g–i, Flow cytometric quantification of LTR expression on Aldefluor (ALD)+ (n = 3 per group) (g) or CD44high MDA-MB-231 cells (n = 4 per group) (h) as well as CD49f+/high 4T1 cells (n = 4 per group) (i). j–l, Sorted LTR+ or LTR− MMTV-PyMT tumour cells were plated in non-attachment conditions followed by sphere quantification at day 10 post-seeding (technical replicate n = 10 per group of biological duplicates) (j) or 103 cells grafted onto the mammary gland of Rag1-null mice for analysis of tumour formation potential. Tumour burden was determined by weighing (n = 8 per group) after 3 weeks (k) and representative image of tumours is shown (l). Statistical analysis by two-sided t-test (a, h–k) and two-way ANOVA (b). Data are represented as mean ± s.e.m. NS, not significant; *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Figure 8 LTs promote stemness within the total cancer cell population by specifically promoting proliferation of MICs.
a, In vitro passaging (P indicates passage number) in non-adherent conditions of sorted CD24+CD90+ MICs and CD24+CD90− non-MICs (n = 4 per group for P0+P1 and n = 3 per group for P2+P3). Quantification was performed by determination of percentage of remaining cell number after 7–10 days. b, Flow cytometric quantification of 3-day LT-treated 4T1 cells for frequency of highly tumorigenic CD49fhigh cells (n = 6). c, Quantification of western blots for ERK1/2 phosphorylation of MMTV-PyMT cells following LTB4 (left) or LTC/D/E4 (right) stimulation relative to α-vinculin as shown in Fig. 3i (n = 2 per time point except n = 9 (30 min LTB4)). d, Dot blot and quantification of ERK1/2 phosphorylation in MDA-MB-231 cells after 3 h stimulation with LTB4 measured by R&D Proteome Profiler Human Phospho-Kinase Array (ARY003B; one-membrane array). e, Flow cytometric quantification of LTR expression of sorted LTR-reduced 4T1 cells (n = 3 per group). f, g, Representative analysis and quantification of western blots for total ERK1/2 and ERK1/2 phosphorylation relative to α-vinculin of unsorted 4T1 cells or 4T1 cells sorted for LTR negativity (n = 2 per group). h–k, Analysis and quantification of western blot for total ERK1/2 and ERK1/2 phosphorylation relative to α-vinculin of 4T1 cells following LTB4 (h, i) or LTC/D/E4 (j, k) stimulation in the presence of BLT2 inhibitor LY255283 or CysLT2 inhibitor BAY-u9773, respectively (one time series). Dotted lines in indicate the control level of ERK1/2 phosphorylation. The decrease of ERK1/2 phosphorylation observed after 5–15 min when adding both leukotrienes and their receptor inhibitors is due to the increase in ethanol concentration. Data are shown as ERK1/2 phosphorylation recovery and increase from 5 to 45 min after stimulation (i, k). l, Flow cytometric quantification of 3-day LTC/D/E4-treated MDA-MB-231 cells for frequency of LTR+ cells (n = 4 per group). m, Three-day LT-treated MMTV-PyMT cells in adherent culture were analysed for BrdU incorporation of CD24+CD90− non-MICs in the additional presence of PD0325901 MEK inhibitor (MEKi; n = 3 per group). DMSO, dimethylsulfoxide treated; EtOH, ethanol treated. Statistical analysis by two-sided t-test (l, m), and one-sided t-test (b). Data are represented as mean ± s.e.m. NS, not significant; *P < 0.05. Blot source data are shown in Supplementary Fig. 1.
Extended Data Figure 9 Analysis of Alox5-null bone marrow chimaeric mice transplanted with primary mammary MMTV-PyMT tumours and failure of Alox5-null neutrophils to support cancer cell metastatic initiation potential.
a, Efficiency of chimaeric mice generation was determined by semi-quantitative PCR analysis of DNA isolated from the bone marrow of lethally irradiated wild-type mice reconstituted with wild-type or Alox5-null bone marrow. A calibration curve of the ratio between the PCR band amplified from the wild-type (WT) and Alox5-null (KO) allele was used to calculate the percentage of bone marrow reconstitution efficiency. Tests of 8 representative Alox5−/− chimaeric mice and 10 controls are shown. Only mice with >80% Alox5-null bone marrow reconstitution were used for further experiments. b–d, Analysis of wild-type and Alox5-null bone marrow chimaeric mice 1.5 months after transplantation with 2 mammary MMTV-PyMT tumours (106 PyMT cells) or tumour-free controls. Representative flow cytometric analysis (b) and quantification of CD11b+Ly6G+ neutrophil presence in the lung (c) (n = 4 (wild type), n = 4 (Alox5−/−), n = 5 (wild-type PyMT), n = 7 (Alox5−/− PyMT) as well as primary mammary tumour burden (n = 6 (wild-type PyMT), n = 9 (Alox5−/− PyMT)) (d). e, f, 5 × 105 luciferase-expressing MMTV-PyMT cells treated with control, wild-type LuN (LuN-WT) or Alox5-deficient neutrophil-derived LuN (LuN-Alox5ko) medium for 3 days in adherent culture were intravenously injected into Rag1-null mice. Quantification of cancer-cell-derived bioluminescence in the lung over time (n = 5 (control), n = 5 (LuN-WT), n = 4 (LuN-Alox5ko)) (e) and representative image is shown (f). Statistical analysis by two-sided t-test. Data are represented as mean ± s.e.m. *P < 0.05, **P < 0.01. Blot source data are shown in Supplementary Fig. 2.
Extended Data Figure 10 Breast cancer cell growth, proliferation and self-renewal are not directly affected by treatment with the Alox5 inhibitor Zil.
a, b, Neutrophils were isolated from the lungs of MMTV-PyMT mammary tumour-bearing mice treated daily with Zil and used to condition culture medium (LuN-Zil) (a). Enzyme-immunoassay analysis of LTB4 levels in control, LuN or LuN-Zil medium (n = 4 (control), n = 4 (LuN), n = 3 (LuN-Zil)) (b). c, d, f–i, Analysis of CD11b+Ly6G+ neutrophils in the lung by flow cytometry (c, f, h) and primary tumour burden (d, g, i) at the time of analysis of Rag1-null mice orthotopically transplanted and intravenously injected with GFP-labelled 105 primary MMTV-PyMT cancer cells (n = 3 (DMSO), n = 9 (PyMT DMSO), n = 7 (PyMT Zil)) (c, d), 105 mouse 4T1 cancer cells (n = 4 (DMSO), n = 5 (4T1 DMSO), n = 7 (4T1 Zil)) (f, g) or 106 human MDA-MB-231 cancer cells (n = 4 (DMSO), n = 6 (MDA231 DMSO), n = 5 (MDA231 Zil)) (h, i), and treated with Zil to complement Fig. 4d–k. e, Determination of in vivo cancer cell proliferation 18 h after intravenous injection of 105 GFP-labelled MMTV-PyMT cancer cells into MMTV-PyMT tumour-bearing, Zil-treated mice by 6 h BrdU pulse and flow cytometric quantification of BrdU+ among GFP+ cancer cells in the lung (n = 3 (PyMT DMSO), n = 4 (PyMT Zil). j, Quantification of mammary tumour load of control (DMSO) or Zil-treated wild-type mice 4–6 weeks after orthotopic transplantation with 106 MMTV-PyMT cells onto the mammary gland. Daily Zil treatment started 1 day prior to mammary tumour engraftment (n = 11 (DMSO), n = 8 (Zil)). k, Flow cytometric quantification of BrdU incorporation after a 3 h pulse of two primary MMTV-PyMT cell preparations and one culture of the mouse 4T1 cell line treated with 1 μM Zil for 24 h in adherent conditions. l, Flow cytometric quantification of frequency of CD24+CD90+ MICs in total MMTV-PyMT cells after 3-day treatment with 1 μM Zil or control DMSO in adherent culture (n = 3 per group). m, Sphere formation of MMTV-PyMT cancer cells in the presence of 1 μM Zil after 7 days (technical replicate n = 8 per group of biological duplicates). Statistical analysis by two-sided t-test (b–d, f–m) and one-sided t-test (e). Data are represented as mean ± s.e.m. NS, not significant, *P < 0.05, ***P < 0.001.
Supplementary information
Supplementary Information
This file contains Supplementary Methods with 2 Supplementary Figures. (PDF 6459 kb)
Supplementary Data
This file contains Supplementary Figures 1-3. (PDF 55687 kb)
Rights and permissions
About this article
Cite this article
Wculek, S., Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015). https://doi.org/10.1038/nature16140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature16140
This article is cited by
-
The oncolytic bacteria-mediated delivery system of CCDC25 nucleic acid drug inhibits neutrophil extracellular traps induced tumor metastasis
Journal of Nanobiotechnology (2024)
-
Cell-cell communication characteristics in breast cancer metastasis
Cell Communication and Signaling (2024)
-
Neutrophils in cancer: dual roles through intercellular interactions
Oncogene (2024)
-
LncRNA Malat1 suppresses pyroptosis and T cell-mediated killing of incipient metastatic cells
Nature Cancer (2024)
-
Cross-talk between disulfidptosis and immune check point genes defines the tumor microenvironment for the prediction of prognosis and immunotherapies in glioblastoma
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.