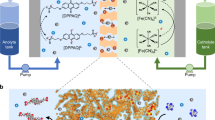
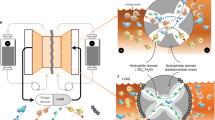
Abstract
For renewable energy sources such as solar, wind, and hydroelectric to be effectively used in the grid of the future, flexible and scalable energy-storage solutions are necessary to mitigate output fluctuations1. Redox-flow batteries (RFBs) were first built in the 1940s2 and are considered a promising large-scale energy-storage technology1,3,4. A limited number of redox-active materials4,5,6,7,8,9,10—mainly metal salts, corrosive halogens, and low-molar-mass organic compounds—have been investigated as active materials, and only a few membrane materials3,5,11,12,13,14, such as Nafion, have been considered for RFBs. However, for systems that are intended for both domestic and large-scale use, safety and cost must be taken into account as well as energy density and capacity, particularly regarding long-term access to metal resources, which places limits on the lithium-ion-based and vanadium-based RFB development15,16. Here we describe an affordable, safe, and scalable battery system, which uses organic polymers as the charge-storage material in combination with inexpensive dialysis membranes, which separate the anode and the cathode by the retention of the non-metallic, active (macro-molecular) species, and an aqueous sodium chloride solution as the electrolyte. This water- and polymer-based RFB has an energy density of 10 watt hours per litre, current densities of up to 100 milliamperes per square centimetre, and stable long-term cycling capability. The polymer-based RFB we present uses an environmentally benign sodium chloride solution and cheap, commercially available filter membranes instead of highly corrosive acid electrolytes and expensive membrane materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011)
Kangro, W. Verfahren zur Speicherung von elektrischer Energie. German patent DE 914 264 (1949)
Yang, Z. et al. Electrochemical energy storage for green grid. Chem. Rev. 111, 3577–3613 (2011)
Alotto, P., Guarnieri, M. & Moro, F. Redox flow batteries for the storage of renewable energy: a review. Renew. Sustain. Energy Rev. 29, 325–335 (2014)
Wang, W. et al. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 23, 970–986 (2013)
Huskinson, B. et al. A metal-free organic–inorganic aqueous flow battery. Nature 505, 195–198 (2014)
Yang, B., Hoober-Burkhardt, L., Wang, F., Surya Prakash, G. K. & Narayanan, S. R. An inexpensive aqueous flow battery for large-scale electrical energy storage based on water-soluble organic redox couples. J. Electrochem. Soc. 161, A1371–A1380 (2014)
Wei, X. et al. TEMPO-based catholyte for high-energy density nonaqueous redox flow batteries. Adv. Mater. 26, 7649–7653 (2014)
Brushett, F. R., Vaughey, J. T. & Jansen, A. N. An all-organic non-aqueous lithium-ion redox flow battery. Adv. Energy Mater. 2, 1390–1396 (2012)
Leung, P. et al. Progress in redox flow batteries, remaining challenges and their applications in energy storage. RSC Adv. 2, 10125–10156 (2012)
Prifti, H., Parasuraman, A., Winardi, S., Lim, T. M. & Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2, 275–306 (2012)
Li, X., Zhang, H., Mai, Z., Zhang, H. & Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 4, 1147–1160 (2011)
Shin, S.-H., Yun, S.-H. & Moon, S.-H. A review of current developments in non-aqueous redox flow batteries: characterization of their membranes for design perspective. RSC Adv. 3, 9095–9116 (2013)
Schwenzer, B. et al. Membrane development for vanadium redox flow batteries. ChemSusChem 4, 1388–1406 (2011)
Barnhart, C. J. & Benson, S. M. On the importance of reducing the energetic and material demands of electrical energy storage. Energy Environ. Sci. 6, 1083–1092 (2013)
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008)
Wang, W. et al. Anthraquinone with tailored structure for a nonaqueous metal–organic redox flow battery. Chem. Commun. 48, 6669–6671 (2012)
Nagarjuna, G. et al. Impact of redox-active polymer molecular weight on the electrochemical properties and transport across porous separators in nonaqueous solvents. J. Am. Chem. Soc. 136, 16309–16316 (2014)
Li, Z. et al. Electrochemical properties of an all-organic redox flow battery using 2,2,6,6-tetramethyl-1-piperidinyloxy and N-methylphthalimide. Electrochem. Solid-State Lett. 14, A171–A173 (2011)
Sukegawa, T., Masuko, I., Oyaizu, K. & Nishide, H. Expanding the dimensionality of polymers populated with organic robust radicals toward flow cell application: synthesis of TEMPO-crowded bottlebrush polymers using anionic polymerization and ROMP. Macromolecules 47, 8611–8617 (2014)
Zhang, H., Zhang, H., Li, X., Mai, Z. & Zhang, J. Nanofiltration (NF) membranes: the next generation separators for all vanadium redox flow batteries (VRBs)? Energy Environ. Sci. 4, 1676–1679 (2011)
Xi, X. et al. Solvent responsive silica composite nanofiltration membrane with controlled pores and improved ion selectivity for vanadium flow battery application. J. Power Sources 274, 1126–1134 (2015)
Zhou, X., Zhao, T. S., An, L., Wei, L. & Zhang, C. The use of polybenzimidazole membranes in vanadium redox flow batteries leading to increased coulombic efficiency and cycling performance. Electrochim. Acta 153, 492–498 (2015)
Ulbricht, M. Advanced functional polymer membranes. Polymer 47, 2217–2262 (2006)
Janoschka, T., Hager, M. D. & Schubert, U. S. Powering up the future: radical polymers for battery applications. Adv. Mater. 24, 6397–6409 (2012)
Nishide, H., Koshika, K. & Oyaizu, K. Environmentally benign batteries based on organic radical polymers. Pure Appl. Chem. 81, 1961–1970 (2009)
Imabayashi, S.-I., Kitamura, N., Tazuke, S. & Tokuda, K. Substituent effects on electrochemical reduction of viologen dimer and trimer with ethylene spacer. J. Electroanal. Chem. 239, 397–403 (1988)
Imabayashi, S.-I., Kitamura, N., Tazuke, S. & Tokuda, K. The role of intramolecular association in the electrochemical reduction of viologen dimers and trimers. J. Electroanal. Chem. 243, 143–160 (1988)
Weber, A. Z. et al. Redox flow batteries: a review. J. Appl. Electrochem. 41, 1137–1164 (2011)
Park, Y. S., Lee, E. J., Chun, Y. S., Yoon, Y. D. & Yoon, K. B. Long-lived charge-separation by retarding reverse flow of charge-balancing cation and zeolite-encapsulated Ru(bpy)3 2+ as photosensitized electron pump from zeolite framework to externally placed viologen. J. Am. Chem. Soc. 124, 7123–7135 (2002)
Wagner, M., Pietsch, C., Tauhardt, L., Schallon, A. & Schubert, U. S. Characterization of cationic polymers by asymmetric flow field-flow fractionation and multi-angle light scattering—a comparison with traditional techniques. J. Chromatogr. A 1325, 195–203 (2014)
Treimer, S., Tang, A. & Johnson, D. C. A consideration of the application of Koutecký–Levich plots in the diagnoses of charge-transfer mechanisms at rotated disk electrodes. Electroanalysis 14, 165–171 (2002)
Cañas, A., Ariza, M. J. & Benavente, J. A comparison of electrochemical and electrokinetic parameters determined for cellophane membranes in contact with NaCl and NaNO3 solutions. J. Colloid Interface Sci. 246, 150–156 (2002)
Zhang, M., Moore, M., Watson, J. S., Zawodzinski, T. A. & Counce, R. M. Capital cost sensitivity analysis of an all-vanadium redox-flow battery. J. Electrochem. Soc. 159, A1183–A1188 (2012)
Viswanathan, V. et al. Cost and performance model for redox flow batteries. J. Power Sources 247, 1040–1051 (2014)
Minke, C. & Turek, T. Economics of vanadium redox flow battery membranes. J. Power Sources 286, 247–257 (2015)
Oh, S. H. et al. A metal-free and all-organic redox flow battery with polythiophene as the electroactive species. J. Mater. Chem. A 2, 19994–19998 (2014)
Acknowledgements
We acknowledge the European Regional Development Fund for Thuringia (EFRE), the Thüringer Aufbaubank (TAB), the Thuringian Ministry for Economic Affairs, Science and Digital Society (TMWWdG), and the Fonds der Chemischen Industrie for financial support. We thank J. Stammer, C. Oder, K. Wolkersdörfer, C. Stolze, C. Schmerbauch, B. Häupler, M. Wagner, T. Buś, A. Ignaszak, and F. Schacher for their assistance and comments.
Author information
Authors and Affiliations
Contributions
T.J., M.D.H., and U.S.S. conceived the studies. N.M., C.F., and T.J. contributed to performing all electrochemical experiments and interpreting the results. S.M. and H.H. performed synthesis under the supervision of T.J. The test cell was designed by U.M. All authors discussed the results and commented on the manuscript. T.J., C.F., M.D.H., and U.S.S wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
U.S.S. is associated with JenaBatteries GmbH as a scientific advisor and T.J., M.D.H. and U.S.S. are co-founders of the company. The intellectual property based upon the present data will be transferred from the Friedrich Schiller University Jena to the JenaBatteries GmbH.
Extended data figures and tables
Extended Data Figure 1 Schematic representation of the synthesis of redox-active polymers.
The cathode material P1 and anode material P2 were prepared by free radical polymerization and subsequent polymer-analogous oxidation and functionalization, respectively. ABCVA, 4,4′-azobis(4-cyanovaleric acid); AIBN, azobisisobutyronitrile; DMSO, dimethyl sulfoxide.
Extended Data Figure 2 Rheogram of redox-active polymers.
The flow behaviour of aqueous solutions of P1 and P2 was studied at 20 °C under continuous shear in sodium chloride solution (1 mol l−1) using a double-gap measuring system in a rotational rheometer. The concentrations of the polymers correspond to a charge-storage capacity of about 10 A h l−1.
Extended Data Figure 3 Test set-up for a polymer-based RFB.
a, Photograph of a laboratory set-up (5-cm2 test cell, peristaltic pump, and electrolyte reservoirs) used for charging/discharging experiments. b, Exploded-view drawing of the 5-cm2 test cell. c, Colour change of the polymer solutions upon charging and discharging.
Extended Data Figure 4 Crossover studies on dialysis membrane.
a, Time-dependent NaCl concentration of a chamber filled with deionized water that is separated from a NaCl feed solution (1 mol l−1) by a dialysis membrane. Salt permeability was determined to be Ps = (9.3 ± 0.1) × 10−5 cm s−1. b, Time-dependent P1 concentration of an RFB test cell compartment filled with NaCl solution (2 mol l−1) that is separated from a P1 feed solution (60 mg ml−1) by a membrane. We determined the maximum polymer permeability from the linear part of the diffusion graph to be Ppolymer,max = (3.2 ± 0.3) × 10−7 cm s−1 (diffusion coefficient Dpolymer,max = (1.3 ± 0.1) × 10−7 cm2 min−1). The minimum membrane selectivity Smin = 290. c, Time-dependent change in P2 concentration of an RFB test cell compartment filled with NaCl solution (2 mol l−1) that is separated from a P2 feed solution (40 mg ml−1) by a dialysis membrane. Ppolymer,max = (3.3 ± 0.4) × 10−8 cm s−1; Dpolymer,max = (1.4 ± 0.2) × 10−8 cm2 min−1; Smin = 2,830. All crossover experiments were conducted with a cellulose-based dialysis membrane (MWCO = 6,000 g mol−1) at 25 °C. In b and c, the slopes of the fit lines correspond to  in
in  (see Methods). d, The number-weighted distributions of hydrodynamic radii (〈 Rh〉n,app) of P1 and P2 determined by dynamic light scattering reveal mean radii of approximately 2 nm (5 g l−1 in 0.1 mol l−1 NaCl solution). Inset, a comparison of the intensity- and number-weighted distributions of P1 shows the presence of aggregates (4.6 nm and 184 nm).
(see Methods). d, The number-weighted distributions of hydrodynamic radii (〈 Rh〉n,app) of P1 and P2 determined by dynamic light scattering reveal mean radii of approximately 2 nm (5 g l−1 in 0.1 mol l−1 NaCl solution). Inset, a comparison of the intensity- and number-weighted distributions of P1 shows the presence of aggregates (4.6 nm and 184 nm).
Extended Data Figure 5 Cyclic voltammogram of electrolytes after 10,000 cycles.
a, b, The cyclic voltammogram (in water with 0.1 mol l−1 NaCl; scan rate of 200 mV s−1) of samples taken from the anolyte (a) and catholyte (b) after repeated charging/discharging; solid lines and dashed lines correspond to the reductive and oxidative range, respectively, of the cyclic voltammogram.
Extended Data Figure 6 Electrochemical analysis of P1 and P2.
a, Cyclic voltammogram of the oxidation process of P1 (2.5 × 10−3 mol l−1 in water with 0.1 mol l−1 NaCl; scan rate of 200 mV s−1). b, Cyclic voltammogram of the first reduction process of P2 (5.2 × 10−3 mol l−1 in water with 0.1 mol l−1 NaCl; scan rate of 200 mV s−1). c, Ultraviolet–visual spectroelectrochemistry of P2 at different applied potentials (as indicated) during consecutive reduction and after subsequent re-oxidation (10−4 mol l−1 in water with 0.1 mol l−1 NaCl). The single-reduced species (Viol+•) is visible at −450 mV (orange line) with the formation of three distinct bands at 365 nm, 530 nm, and 900 nm, which disappear upon further reduction towards the double-reduced species (Viol0). The shapes and positions of the emerging bands strongly suggest the formation of radical cation dimers27. Re-oxidation at 200 mV (dotted line) restores the initial spectrum.
Extended Data Figure 7 Toxicity tests of the redox-active polymers.
a, The viability of L929 mouse cells was tested in the presence of redox-active compounds according to ISO10993-5. Cell viability below 70% is considered indicative of cytotoxicity. The negative control was standardized as 0% of metabolism inhibition and referred as 100% viability. Two vanadium salts in redox states commonly found in vanadium-based RFBs and two widely used cationic polymers were used as a reference. Poly(l-lysine), PLL, is a commercial food preservative and branched poly(ethylene imine), bPEI, is used in the paper-making industry and as a flocculating agent. Although P1 and P2 show cytotoxic effects at concentrations >50 µg ml−1—with P1 being less toxic than P2—the vanadium salts and the cationic polymers reveal cytotoxic effects at lower concentrations (VOSO4 > 5 µg ml−1, VCl3 > 10 µg ml−1, bPEI > 5 µg ml−1, PLL > 25 µg ml−1). Data are expressed as mean values and error bars represent the standard deviation of three determinations. b, We quantified the cell-membrane damaging properties of the polymers by analysing the haemoglobin release from erythrocytes (indicated by the numbers associated with each bar). Data are expressed as mean values and error bars represent the standard deviation of triplicates of three different blood samples per concentration. Because a haemoglobin release (haemolysis rate) less than 2% is considered non-haemolytic, P1 and P2 as well as the reference compounds show no membrane damaging behaviour. Hence, the cell toxic effects do not originate from damage to the cell membrane, but from reactions within the cell. Because the cell uptake via diffusive processes of polymers is hindered in comparison to ‘small’ inorganic ions, P1 and P2 possess lower cytotoxicity. These tests provide some insight into the toxicity, but long-term ecotoxicity tests and animal testing are required to fully evaluate the impact of the redox-active polymers on wildlife and plants.
Extended Data Figure 8 RDE measurements of P1 and analysis.
a, Voltammograms of P1 (2.5 × 10−3 mol l−1 in water with 0.1 mol l−1 NaCl; scan rate of 5 mV s−1) at different rotation speeds (as indicated) from 400 r.p.m. to 3,600 r.p.m. (The arrow indicates the direction of potential scanning). b, Levich plot from the obtained limiting currents; application of Levich equation yields a diffusion coefficient D = (7.0 ± 0.5) × 10−8 cm2 s−1. c, Koutecký–Levich plot for different overpotentials η (as indicated) yielding the mass-transfer-independent current ik (as ω−1/2 → 0, ik = i). d, Tafel plot yielding k0 = (4.5 ± 0.1) × 10−4 cm s−1 and α = 0.68 ± 0.03 (see Methods).
Extended Data Figure 9 RDE measurements of P2 and analysis.
a, Voltammograms of P2 (5.2 × 10−3 mol l−1 in water with 0.1 mol l−1 NaCl; scan rate 5 mV s−1) at different rotation speeds (as indicated) from 100 r.p.m. to 4,900 r.p.m.; substantial changes of the limiting current, necessary for reasonable analysis, are observed only at rotation speeds >1,200 r.p.m. (The arrow indicates the direction of potential scanning). b, Levich plot for currents between the first and second steps of the two-step process; the Levich equation was applied only for high rotation rates, that is, in the region of substantial changes of the limiting current, yielding a diffusion coefficient D = (7.6 ± 0.9) × 10−7 cm2 s−1. The fit curve and its slope correspond to the Levich equation (see Methods). c, Plot of i−1 versus ω−1/2 for high negative overpotentials η (as indicated) yielding 1/ik (as ω−1/2 → 0, 1/ik = 1/i). d, Plot of log|1/ik| versus η1 (overpotential with respect to the first step) yielding −log|2FAk0c0| (log|1/ik| for η1 = 0), which allows us to determine k0 = (9 ± 2) × 10−5 cm s−1. The error bars represent error that originates from the linear regression analysis of c.
Supplementary information
Supplementary Data
This file contains the ChemDraw structure 1 in Figure 1a. (CDX 5 kb)
Supplementary Data
This file contains the ChemDraw structure 2 in Figure 1a. (CDX 6 kb)
Supplementary Data
This file contains the ChemDraw structure in Figure 1b. (CDX 9 kb)
Rights and permissions
About this article
Cite this article
Janoschka, T., Martin, N., Martin, U. et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 527, 78–81 (2015). https://doi.org/10.1038/nature15746
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature15746
This article is cited by
-
Perspective of alkaline zinc-based flow batteries
Science China Chemistry (2024)
-
A solubility limited pyrene-4,5,9,10-tetraone-based covalent organic framework for high-performance aqueous zinc-organic batteries
Nano Research (2024)
-
Organic catalyst opens way to energy-efficient chlorine production
Nature (2023)
-
The role of the electrolyte in non-conjugated radical polymers for metal-free aqueous energy storage electrodes
Nature Materials (2023)
-
Thianthrene polymers as 4 V-class organic mediators for redox targeting reaction with LiMn2O4 in flow batteries
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.