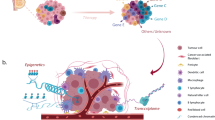
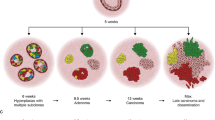
Abstract
Most human breast cancers have diversified genomically and biologically by the time they become clinically evident1,2,3. Early events involved in their genesis and the cellular context in which these events occur have thus been difficult to characterize. Here we present the first formal evidence of the shared and independent ability of basal cells and luminal progenitors, isolated from normal human mammary tissue and transduced with a single oncogene (KRASG12D), to produce serially transplantable, polyclonal, invasive ductal carcinomas within 8 weeks of being introduced either subrenally or subcutaneously into immunodeficient mice4. DNA barcoding5,6 of the initial cells revealed a dramatic change in the numbers and sizes of clones generated from them within 2 weeks, and the first appearance of many ‘new’ clones in tumours passaged into secondary recipients. Both primary and secondary tumours were phenotypically heterogeneous and primary tumours were categorized transcriptionally as ‘normal-like’. This system challenges previous concepts that carcinogenesis in normal human epithelia is necessarily a slow process requiring the acquisition of multiple driver mutations. It also presents the first description of initial events that accompany the genesis and evolution of malignant human mammary cell populations, thereby contributing new understanding of the rapidity with which heterogeneity in their properties can develop.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Data deposits
Final transcriptome data has been deposited in the European Genome-phenome Archive (www.ebi.ac.uk/ega) under accession number EGAS00001001310.
References
Stephens, P. J. et al. Oslo Breast Cancer Consortium (OSBREAC). The landscape of cancer genes and mutational processes in breast cancer. Nature 486, 400–404 (2012)
Sørlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA 98, 10869–10874 (2001)
Curtis, C. et al. METABRIC Group. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012)
Eirew, P. et al. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nature Med. 14, 1384–1389 (2008)
Nguyen, L. V. et al. DNA barcoding reveals diverse growth kinetics of human breast tumour subclones in serially passaged xenografts. Nature Commun. 5, 5871 (2014)
Nguyen, L. V. et al. Clonal analysis via barcoding reveals diverse growth and differentiation of transplanted mouse and human mammary stem cells. Cell Stem Cell 14, 253–263 (2014)
Kannan, N. et al. Glutathione-dependent and -independent oxidative stress-control mechanisms distinguish normal human mammary epithelial cell subsets. Proc. Natl Acad. Sci. USA 111, 7789–7794 (2014)
Kannan, N. et al. The luminal progenitor compartment of the normal human mammary gland constitutes a unique site of telomere dysfunction. Stem Cell Rep. 1, 28–37 (2013)
Eirew, P. et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature 518, 422–426 (2015)
Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27, 1160–1167 (2009)
Paquet, E. R. & Hallett, M. T. Absolute assignment of breast cancer intrinsic molecular subtype. J. Natl Cancer Inst. 107, 357 (2015)
Keller, P. J. et al. Defining the cellular precursors to human breast cancer. Proc. Natl Acad. Sci. USA 109, 2772–2777 (2012)
Kreso, A. et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 339, 543–548 (2013)
Bhang, H. E. et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nature Med. 21, 440–448 (2015)
Logan, A. C. et al. Factors influencing the titer and infectivity of lentiviral vectors. Hum. Gene Ther. 15, 976–988 (2004)
Imren, S. et al. High-level β-globin expression and preferred intragenic integration after lentiviral transduction of human cord blood stem cells. J. Clin. Invest. 114, 953–962 (2004)
Eirew, P., Stingl, J. & Eaves, C. J. Quantitation of human mammary epithelial stem cells with in vivo regenerative properties using a subrenal capsule xenotransplantation assay. Nature Protocols 5, 1945–1956 (2010)
Lakhani, S. R., Ellis, I. O., Schnitt, S. J., Tan, P. H. & van de Vijver, M. J. WHO Classification of Tumours of the Breast 4th edn, Ch. 2 (World Health Organization, 2012)
Tsuda, H., Akiyama, F., Kurosumi, M., Sakamoto, G. & Watanabe, T. ; Japan National Surgical Adjuvant Study of Breast Cancer (NSAS-BC) Pathology Section. Establishment of histological criteria for high-risk node-negative breast carcinoma for a multi-institutional randomized clinical trial of adjuvant therapy. Jpn. J. Clin. Oncol. 28, 486–491 (1998)
Elston, C. W. & Ellis, I. O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19, 403–410 (1991)
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009)
Butterfield, Y. S. et al. JAGuaR: junction alignments to genome for RNA-seq reads. PLoS One 9, e102398 (2014)
Li, H. et al. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009)
Gascard, P. et al. Epigenetic and transcriptional determinants of the human breast. Nature Commun. 6, 6351 (2015)
Acknowledgements
We thank D. Wilkinson, G. Edin and M. Hale for technical support, E. Bovill, J. Boyle, S. Bristol, P. Gdalevitch, A. Seal, J. Sproul and N. van Laeken for access to discarded reduction mammoplasty tissue, T. Nielsen and N. Poulin for discussions, the Centre for Translational and Applied Genomics (BC Cancer Agency) for assistance with IHC, and T. MacDonald for assistance with rodent husbandry. This work was supported by grants from the Canadian Cancer Society Research Institute, the Canadian Breast Cancer Foundation and the Canadian Breast Cancer Research Alliance. L.V.N. received a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research (CIHR), and N.K. was supported by a MITACS Elevate Fellowship. T.O. was supported by a Molecular Oncologic Pathology Fellowship from CIHR and the Terry Fox Foundation, and by grants from the Sumitomo Life Welfare and Culture Foundation, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Takashi Tsuruo Memorial Fund. S.A. is supported by a Canada Research Chair.
Author information
Authors and Affiliations
Contributions
L.V.N., D.P. and C.J.E. designed the project, drafted the manuscript and were assisted by S.L., C.L.C., W.K. and S. Balani in performing the experiments. M.M. and M.H. oversaw the generation of sequence data, and L.V.N., D.P., A.C. and M.B. analysed it. All authors contributed to the interpretation of the results, and read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Quantification of human cells containing different vector reporters in tumours derived from triply transduced starting populations.
a, Lentiviral constructs used. CBR-Luc, click beetle red luciferase. b, Frequencies of donor samples producing at least one tumour subrenally from BCs or LPs exposed to different combinations of oncogene-encoding vectors. c, Representative FACS profiles of a cell suspension prepared from a tumour produced from cells transduced with all three genes and sorted for human EpCAM and HLA using human-specific antibodies. d, Percentage of cells expressing different lentiviral reporters in cells maintained in vitro for 72 h after transduction, and in primary and secondary tumours (G, GFP; Y, YFP; mCh, mCherry). e, Frequencies of donor samples producing at least one tumour under various transplantation conditions using BCs or LPs transduced with KRASG12D. f, Percentages of human cells in BC- and LP-derived tumours detected by FACS on the basis of their expression of human EpCAM and/or HLA.
Extended Data Figure 2 Molecular characterization of the tumours.
a, Examples of PCR evidence of all three vectors in DNA extracts obtained from a subset of tumours analysed with vector-specific primers. b, A representative Sanger sequencing chromatograph showing the expected point mutations in the tumour cells analysed. c, PCR evidence of the three vectors in FACS-purified doubly and triply transduced cells. d, e, Representative images of H&E- and IHC-stained sections of primary tumours (d, arising from cells transplanted subcutaneously) and secondary tumours (e, all arising subcutaneously) derived from either BCs or LPs. Scale bar, 50 μm. f, Relative expression (negative ΔCt values, mean ± s.e.m.) of gene transcripts typically associated with mesenchymal/basal or epithelial/luminal phenotypes, or associated with proliferation and cell growth.
Extended Data Figure 3 Threshold set for detection of barcoded clones for the two sequencing runs from which barcode data were acquired.
a, The relationship between the fractional read value (FRV) and the number of cells per clone. Spiked-in controls only and spiked-in controls added to experimental samples are shown as red and grey points, respectively. The shaded grey box represents distribution of false positive barcodes. b, Sensitivity and specificity data for controls compared with experimental samples for different sized clones.
Extended Data Figure 4 Clonal analyses of primary barcoded tumours.
a, Numbers of clones and frequencies of T-CFCs in primary tumours. b, Relative clone size distributions for individual primary tumours grouped by the cell type initially manipulated and the oncogene(s) used. Each column represents a single tumour. Each rectangle represents one clone. Its relative clone size is indicated by the shade of green, and its proportional contribution within each tumour is indicated by its length on the y axis.
Extended Data Figure 5 Clonal analyses of secondary barcoded tumours.
a, Numbers of clones and frequencies of T-CFCs in secondary tumours. b, Relative clone size distributions for individual secondary tumours grouped by the cell type initially manipulated. Each column represents a single tumour. Each rectangle represents one clone. Its relative clone size is indicated by the shade of green, and its proportional contribution within each tumour is indicated by its length on the y axis. c, Clonal landscape of replicate secondary tumours generated from single primary tumours in two separate experiments. Clones present in sibling tumours are shown above one another and unique clones are shown in the same horizontal bar. Increasing clone sizes are indicated by a grey intensity scale. d, Numbers of clones and T-CFC frequencies of combined primary and secondary tumours.
Extended Data Figure 6 Clonal analyses of transduced cells transplanted subrenally after 2 weeks in vivo.
a, Number of clones and frequency of CFCs in xenografts of transduced cells assessed after 2 weeks in vivo. b, Relative clone size distributions of individual 2-week transplants grouped by the cell type initially manipulated and the oncogene(s) used. Each column represents a single transplant. Each rectangle represents one clone. Its relative clone size is indicated by the shade of green, and its proportional contribution within each tumour is indicated by its length on the y axis.
Supplementary information
Supplementary Table 1
This file contains Luciferase activity values. (XLSX 11 kb)
Supplementary Table 2
This file contains lists of differentially expressed genes. (XLSX 113 kb)
Supplementary Table 3
This file shows Antibodies used for FACS and IHC analyses. (XLSX 9 kb)
Supplementary Table 4
This file contains Primers used for RT-qPCR. (XLSX 8 kb)
Supplementary Table 5
This file contains quality control report of RNA-seq data. (XLSX 15 kb)
Rights and permissions
About this article
Cite this article
Nguyen, L., Pellacani, D., Lefort, S. et al. Barcoding reveals complex clonal dynamics of de novo transformed human mammary cells. Nature 528, 267–271 (2015). https://doi.org/10.1038/nature15742
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature15742
This article is cited by
-
Experimental and spontaneous metastasis assays can result in divergence in clonal architecture
Communications Biology (2023)
-
Know thy cells: commonly used triple-negative human breast cancer cell lines carry mutations in RAS and effectors
Breast Cancer Research (2022)
-
De novo and cell line models of human mammary cell transformation reveal an essential role for Yb-1 in multiple stages of human breast cancer
Cell Death & Differentiation (2022)
-
Mastering the use of cellular barcoding to explore cancer heterogeneity
Nature Reviews Cancer (2022)
-
Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression
British Journal of Cancer (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.