Abstract
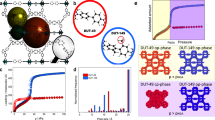
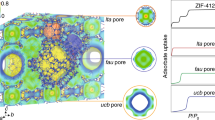
Metal-organic frameworks (MOFs) have a high internal surface area and widely tunable composition1,2, which make them useful for applications involving adsorption, such as hydrogen, methane or carbon dioxide storage3,4,5,6,7,8,9. The selectivity and uptake capacity of the adsorption process are determined by interactions involving the adsorbates and their porous host materials. But, although the interactions of adsorbate molecules with the internal MOF surface10,11,12,13,14,15,16,17 and also amongst themselves within individual pores18,19,20,21,22 have been extensively studied, adsorbate–adsorbate interactions across pore walls have not been explored. Here we show that local strain in the MOF, induced by pore filling, can give rise to collective and long-range adsorbate–adsorbate interactions and the formation of adsorbate superlattices that extend beyond an original MOF unit cell. Specifically, we use in situ small-angle X-ray scattering to track and map the distribution and ordering of adsorbate molecules in five members of the mesoporous MOF-74 series along entire adsorption–desorption isotherms. We find in all cases that the capillary condensation that fills the pores gives rise to the formation of ‘extra adsorption domains’—that is, domains spanning several neighbouring pores, which have a higher adsorbate density than non-domain pores. In the case of one MOF, IRMOF-74-V-hex, these domains form a superlattice structure that is difficult to reconcile with the prevailing view of pore-filling as a stochastic process. The visualization of the adsorption process provided by our data, with clear evidence for initial adsorbate aggregation in distinct domains and ordering before an even distribution is finally reached, should help to improve our understanding of this process and may thereby improve our ability to exploit it practically.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
25 November 2015
Minor updates were made to the Acknowledgements.
References
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Edn 43, 2334–2375 (2004)
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013)
Rosi, N. L. et al. Hydrogen storage in microporous metal-organic frameworks. Science 300, 1127–1129 (2003)
Dincă, M. et al. Hydrogen storage in a microporous metal-organic framework with exposed Mn2+ coordination sites. J. Am. Chem. Soc. 128, 16876–16883 (2006)
Farha, O. K. et al. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nature Chem. 2, 944–948 (2010)
Holst, J. R. & Cooper, A. I. Ultrahigh surface area in porous solids. Adv. Mater. 22, 5212–5216 (2010)
Makal, T. A., Li, J., Lu, W. & Zhou, H. Methane storage in advanced porous materials. Chem. Soc. Rev. 41, 7761–7779 (2012)
Deng, H. et al. Large-pore apertures in a series of metal-organic frameworks. Science 336, 1018–1023 (2012)
Nugent, P. et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 495, 80–84 (2013)
Rowsell, J. L. C., Spenser, E. C., Eckert, J., Howard, J. A. K. & Yaghi, O. M. Gas adsorption sites in a large-pore metal-organic framework. Science 309, 1350–1354 (2005)
Vaidhyanathan, R. et al. Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid. Science 330, 650–653 (2010)
Yang, S. et al. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host. Nature Chem. 4, 887–894 (2012)
Serre, C. et al. Role of solvent-host interactions that lead to very large swelling of hybrid frameworks. Science 315, 1828–1831 (2007)
Rabone, J. et al. An adaptable peptide-based porous material. Science 329, 1053–1057 (2010)
Scherb, C., Koehn, R. & Bein, T. Sorption behavior of an oriented surface-grown MOF-film studied by in situ X-ray diffraction. J. Mater. Chem. 20, 3046–3051 (2010)
Bureekaew, S. et al. Control of interpenetration for tuning structural flexibility impacts on sorption properties. Angew. Chem. Int. Edn 49, 7660–7664 (2010)
Sato, H. et al. Self-accelerating CO sorption in a soft nanoporous crystal. Science 343, 167–170 (2014)
Inagaki, S., Guan, S., Ohsuna, T. & Terasaki, O. An ordered mesoporous organosilica hybrid material with a crystal-like wall structure. Nature 416, 304–307 (2002)
Zhao, D. et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998)
Joo, S. H. et al. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 412, 169–172 (2001)
Muroyama, N. et al. Argon adsorption on MCM-41 mesoporous crystal studied by in situ synchrotron powder X-ray diffraction. J. Phys. Chem. C 112, 10803–10813 (2008)
Miyasaka, K., Neimark, A. V. & Terasaki, O. Density functional theory of in-situ synchrotron powder X-ray diffraction on mesoporouscrystals: argon adsorption on MCM-41. J. Phys. Chem. C 113, 791–794 (2009)
Rosi, N. L. et al. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units. J. Am. Chem. Soc. 127, 1504–1518 (2005)
Dietzel, P. D. C., Blom, R. & Fjellvåg, H. Base-induced formation of two magnesium metal-organic framework compounds with a bifunctional tetratopic ligand. Eur. J. Inorg. Chem. 2008, 3624–3632 (2008)
Lin, L. C. et al. Understanding CO2 dynamics in metal-organic frameworks with open metal sites. Angew. Chem. Int. Edn 52, 4410–4413 (2013)
Bon, V. et al. In situ monitoring of structural changes during the adsorption on flexible porous coordination polymers by X-ray powder diffraction: instrumentation and experimental results. Microporous Mesoporous Mater. 188, 190–195 (2014)
Gor, G. Y. et al. Adsorption of n-pentane on mesoporous silica and adsorbent deformation. Langmuir 29, 8601–8608 (2013)
Marra, G. L. et al. Cation location in dehydrated Na-Rb-Y zeolite: an XRD and IR study. J. Phys. Chem. B 101, 10653–10660 (1997)
Petříček, V., Dušek, M. & Palatinus, L. Crystallographic computing system JANA2006: general features. Zeitschrift Kristal. Crystalline Mater. 229, 345–352 (2006)
Momma, K. & Izumi, K. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011)
Acknowledgements
The authors acknowledge K. Ito, K. Sasaki, M. Kuribayashi and N. Muroyama (Rigaku America and Japan) and K. Nakai (Japan Bel) for technical support; N. Fujita and T. Nishimatsu (Tohoku University, Japan), H. Furukawa and Y. Zhang (University of California at Berkeley, USA) for their input; and A. Sawada (Kyoto University, Japan) for advice in designing the gas cell. Financial support was provided by WCU/BK21+ (to H.S.C., K.M., J.K.K., O.M.Y. and O.T.); HIMC of Global Frontier Project (2013M3A6B1078884) funded by the Ministry of Science, ICT and Future Planning and Korea Center for Artificial Photosynthesis (to J.K.K.); Berzelii Centre EXSELENT on Porous Materials (to O.T.); and BASF (Ludwigshafen, Germany) (to O.M.Y.). H.D. and Z.D. were supported by the 1000 Talent Plan of China, National Natural Science Foundation of China (21471118) and National Key Basic Research Program of China (2014CB239203). A.V.N. acknowledges support from the NSF ERC ‘Structured Organic Particulate Systems’.
Author information
Authors and Affiliations
Contributions
O.T., K.M. and J.K.K. designed and set up the experimental system. O.T. and O.M.Y. designed and led the project. H.S.C., K.M. and H.D. performed the SAXS experiments. H.D., Z.D. and M.C. prepared samples. A.V.N. contributed discussion of the gas adsorption–desorption process. H.D., H.S.C., J.K.K., O.M.Y. and O.T. prepared the first version of the manuscript and all authors contributed to the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 The five stages of gas adsorption in IRMOF-74s.
The five different adsorption stages are indicated in red at the top of the figure and their boundaries demarcated throughout all panels by grey dashed lines. a, The measured Ar adsorption by IRMOF-74-V-hex is shown; it can be compared against relevant SAXS profile features of IRMOF-74, measured as a function of Ar pressure, that are shown in the other panels. b, The appearance and disappearance of the broad peak indicates the formation of extra adsorption domains over pores (aggregation, red) and the even distribution of adsorbates (homogenization, blue). c, Intensity of 1 superlattice reflection, appearing as stage 3 turns to stage 4 (organization, green) and disappearing at the end of stage 4 (homogenization, blue). d, Change in the unit-cell parameter a of IRMOF-74. e, Change in the line-profile width of IRMOF-74.
superlattice reflection, appearing as stage 3 turns to stage 4 (organization, green) and disappearing at the end of stage 4 (homogenization, blue). d, Change in the unit-cell parameter a of IRMOF-74. e, Change in the line-profile width of IRMOF-74.
Supplementary information
Supplementary Information
This file contains detailed instrumental set-up and results of in-situ SAXS measurements of the Ar, CO2 and N2 adsorption in IRMOF-74-III, -IV, -V, -V-hex and VII, Supplementary Figures 1-57 and Supplementary Tables 1-25. (PDF 8782 kb)
Collective behavior of adsorbate exemplified by Ar distribution along the isotherm in IRMOF-74-V-hex
This video shows systematic change of electron density map of Ar in IRMOF-74-V-hex during adsorption-desorption process. (MP4 6998 kb)
Rights and permissions
About this article
Cite this article
Sung Cho, H., Deng, H., Miyasaka, K. et al. Extra adsorption and adsorbate superlattice formation in metal-organic frameworks. Nature 527, 503–507 (2015). https://doi.org/10.1038/nature15734
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature15734
This article is cited by
-
Efficient capture and storage of ammonia in robust aluminium-based metal-organic frameworks
Communications Chemistry (2023)
-
Nanoscale molecular rectifiers
Nature Reviews Chemistry (2023)
-
The Preparation and Application in Adsorptive Removal Hazardous Materials of MOF-Derived Materials
Journal of Inorganic and Organometallic Polymers and Materials (2023)
-
UiO-66 derived nanoporous carbons/electrochemically reduced graphene oxide nanocomposites-based non-enzyme electrochemical sensor towards highly efficient determination of methyl parathion in food samples
Journal of Materials Science (2023)
-
Filling metal–organic framework mesopores with TiO2 for CO2 photoreduction
Nature (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.