Abstract
Postnatal tissue quiescence is thought to be a default state in the absence of a proliferative stimulus such as injury. Although previous studies have demonstrated that certain embryonic developmental programs are reactivated aberrantly in adult organs to drive repair and regeneration1,2,3, it is not well understood how quiescence is maintained in organs such as the lung, which displays a remarkably low level of cellular turnover4,5. Here we demonstrate that quiescence in the adult lung is an actively maintained state and is regulated by hedgehog signalling. Epithelial-specific deletion of sonic hedgehog (Shh) during postnatal homeostasis in the murine lung results in a proliferative expansion of the adjacent lung mesenchyme. Hedgehog signalling is initially downregulated during the acute phase of epithelial injury as the mesenchyme proliferates in response, but returns to baseline during injury resolution as quiescence is restored. Activation of hedgehog during acute epithelial injury attenuates the proliferative expansion of the lung mesenchyme, whereas inactivation of hedgehog signalling prevents the restoration of quiescence during injury resolution. Finally, we show that hedgehog also regulates epithelial quiescence and regeneration in response to injury via a mesenchymal feedback mechanism. These results demonstrate that epithelial–mesenchymal interactions coordinated by hedgehog actively maintain postnatal tissue homeostasis, and deregulation of hedgehog during injury leads to aberrant repair and regeneration in the lung.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Beers, M. F. & Morrisey, E. E. The three R’s of lung health and disease: repair, remodeling, and regeneration. J. Clin. Invest. 121, 2065–2073 (2011)
Herriges, M. & Morrisey, E. E. Lung development: orchestrating the generation and regeneration of a complex organ. Development 141, 502–513 (2014)
Hogan, B. L. et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123–138 (2014)
Blenkinsopp, W. K. Proliferation of respiratory tract epithelium in the rat. Exp. Cell Res. 46, 144–154 (1967)
Breuer, R., Zajicek, G., Christensen, T. G., Lucey, E. C. & Snider, G. L. Cell kinetics of normal adult hamster bronchial epithelium in the steady state. Am. J. Respir. Cell Mol. Biol. 2, 51–58 (1990)
McMahon, A. P., Ingham, P. W. & Tabin, C. J. Developmental roles and clinical significance of hedgehog signaling. Curr. Top. Dev. Biol. 53, 1–114 (2003)
Lum, L. & Beachy, P. A. The Hedgehog response network: sensors, switches, and routers. Science 304, 1755–1759 (2004)
Peng, T. et al. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 500, 589–592 (2013)
Harfe, B. D. et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517–528 (2004)
Bai, C. B., Auerbach, W., Lee, J. S., Stephen, D. & Joyner, A. L. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129, 4753–4761 (2002)
Liu, L. et al. Hedgehog signaling in neonatal and adult lung. Am. J. Respir. Cell Mol. Biol. 48, 703–710 (2013)
Ahn, S. & Joyner, A. L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437, 894–897 (2005)
Li, H. et al. Cre-mediated recombination in mouse Clara cells. Genesis 46, 300–307 (2008)
Foo, S. S. et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124, 161–173 (2006)
Jeong, J., Mao, J., Tenzen, T., Kottmann, A. H. & McMahon, A. P. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 18, 937–951 (2004)
Olson, L. E. & Soriano, P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev. Cell 16, 303–313 (2009)
Olson, L. E. & Soriano, P. PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev. Cell 20, 815–826 (2011)
Buckpitt, A. R., Bahnson, L. S. & Franklin, R. B. Comparison of the arachidonic acid and NADPH-dependent microsomal metabolism of naphthalene and 2-methylnaphthalene and the effect of indomethacin on the bronchiolar necrosis. Biochem. Pharmacol. 35, 645–650 (1986)
Bolaños, A. L. et al. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L978–L990 (2012)
Watkins, D. N. et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 422, 313–317 (2003)
Zemke, A. C. et al. β-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am. J. Respir. Cell Mol. Biol. 41, 535–543 (2009)
Wang, Y. et al. Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Dev. Cell 24, 345–358 (2013)
Rawlins, E. L., Ostrowski, L. E., Randell, S. H. & Hogan, B. L. Lung development and repair: contribution of the ciliated lineage. Proc. Natl Acad. Sci. USA 104, 410–417 (2007)
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010)
Long, F., Zhang, X. M., Karp, S., Yang, Y. & McMahon, A. P. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 128, 5099–5108 (2001)
Lewis, P. M. et al. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell 105, 599–612 (2001)
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007)
Ruzankina, Y. et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 (2007)
Hama, H. et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nature Neurosci. 14, 1481–1488 (2011)
Acknowledgements
The authors appreciate the input of M. Kahn, M. Beers and R. Shah in these studies. The authors are grateful to A. Stout and the Department of Cell and Developmental Biology Microscopy Core for help with imaging. These studies were supported by funds from the National Institutes of Health (HL110942, HL100405, HL087825 to E.E.M). T.P. is supported by the American Heart Association Fellow-to-Faculty Transition Award, Actelion ENTELLIGENCE Award, and K08-HL121146.
Author information
Authors and Affiliations
Contributions
T.P. and E.E.M. designed the overall experimental strategy. T.P. and R.S.K. performed lineage tracing and animal injury experiments. T.P. and D.B.F. performed in vitro BrdU and organoid experiments. T.W. performed right heart catheterization on the animals. S.Z., L.C. and M.M.L. performed histology and immunohistochemistry. T.P., D.B.F., K.S.R., M.P.M. and E.E.M. analysed the data. T.P. and E.E.M. wrote and edited the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Characterization of Hh signalling in the lung.
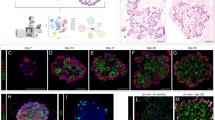
a, A small number of TubbIV+ ciliated cells express GFP in the ShhcreGFP reporter. b, c, Gli1+ Hh activated cells co-label with S100A4, a fibroblast marker. d–g, Lineage tracing of Gli1+ Hh activated cells shows little to no co-localization of GFP+ cells with the airway smooth muscle (d, e) or the vascular smooth muscle of the adjacent pulmonary artery (f, g). h, i, The rare exception occurs in the pulmonary vein where Gli1+ Hh activated cells contribute to the venous smooth muscle that is surrounded by the venous myocardium of the proximal pulmonary vein. j, Gli1+ cells also generate myofibroblasts after fibrotic injury such as that induced by bleomycin. k, Lung Gli1+ cells do not contribute to cells of haematopoietic lineage as marked by CD45. AW, airway; V, blood vessel; PV, pulmonary vein. Scale bars, 100 μm. Images representative of 3 animals with 5 sections examined per animal.
Extended Data Figure 2 Conditional deletion of Shh from the adult airway epithelium increases proliferation in the adjacent mesenchyme.
a, The Scgb1a1cre driver predominantly marks the airway epithelium in the adult lung when crossed with the R26RmTmG reporter. b, Whole-lung messenger RNA transcript analysis reveals efficient deletion of Shh transcripts in the Scgb1a1cre:Shhflox/flox animals compared to controls (Shhflox/flox) (n = 4 animals). c–h, Deletion of Shh from the airway epithelium resulted in an increased expression of proliferative markers, PCNA and phospho-histone H3 (PH3) in the mesenchyme surrounding the airways in Scgb1a1cre:Shhflox/flox mutants (d, f, h, n = 4 animals) versus controls (Scgb1a1Cre:Shhflox/+) (c, e, g, n = 3 animals). AW, airway; V, blood vessel. Scale bars, 100 μm. *P < 0.05. Error bars, mean ± s.d.
Extended Data Figure 3 Conditional deletion of Smo from Pdgfrb-derived mesenchyme increases mesenchymal proliferation at the epithelial–mesenchymal interface and vascular remodelling.
a–d, Deletion of Smo from Pdgfrb-derived mesenchyme resulted in increased expression of proliferative markers, PCNA and PH3 in the mesenchyme at the epithelial-mesenchymal interface of Pdgfrbcre:Smoflox/flox mutants (b, d) versus controls (Pdgfrb Cre:Smoflox/+) (a, c). e–i, Pdgfrbcre:Smoflox/flox:R26RmTmG/+ mutants exhibit increased Ki67+ cells within lineage traced GFP+ cells in the alveoli compared to controls (n = 4 animals per group). j, k, Aged Pdgfrbcre:Smoflox/flox:R26RmTmG/+ mutants (>6 months old) spontaneously develop pulmonary hypertension with increased right ventricular systolic pressure (j, n = 3 animals per group) and right ventricle wall thickness (k, l). Scale bars, 100 μm. *P < 0.05. Error bars, mean ± s.d.
Extended Data Figure 4 Characterization of isolated lung mesenchyme.
a–h, Isolated mesenchymal cells in vitro predominantly express Pdgfra and Pdgfrb (c, d, compared to isotype control a), but not epithelial marker, Epcam (b, compared to isotype control a), endothelial marker, CD31 (f, compared to isotype control e), nor haematopoietic marker, CD45 (h, compared to isotype control g). i, Expression of the constitutively active form of Smo (SmoM2) by 4-OH-tamoxifen induction significantly upregulates Gli1 expression in the isolated lung mesenchyme after 48 h. j, GFP staining of the PdgfraGFP reporter demonstrates that Pdgfra+ cells are expressed broadly in the lung. k, Activation of Smo in isolated lung mesenchymal cells leads to reduced expression of cell cycle progression genes. l, Hh activation of lung mesenchyme with SmoM2 attenuated the proliferation induced by Pdgf-BB ligand in vitro as assayed by BrdU incorporation. m–p, s, Expression of activated Pdgfrb(PdgfrbGOF) within Gli1+ cells resulted in their proliferative expansion. q–s, Concurrent activation of SmoM2 attenuated the proliferative expansion induced by PdgfrbGOF (n = 3 animals per group). Scale bars, 100 μm. Error bars, mean ± s.d.
Extended Data Figure 5 Shh expression is decreased with bleomycin and naphthalene injury to the airway.
a–g, Repetitive bleomycin injury after one month or single-dose naphthalene injury after three days reduced GFP expression in the airways of the ShhcreGFP reporter compared to controls. Data represent n = 2 animals per group with 5 sections analysed per animal, AW, airway. Scale bars, 100 μm. *P < 0.05.
Extended Data Figure 6 Hedgehog modulates mesenchymal response to bleomycin injury.
a–e, Chronic repetitive injury to the lung epithelium with bleomycin over 4 weeks downregulates Gli1 expression in the mesenchyme adjacent to the airways in Gli1lacZ lungs as noted by histochemical staining for β-galactosidase activity (a–d) and as noted using X-gal quantification and qPCR analysis of Shh and Gli1 expression after four weeks of repetitive injury (e, n = 2 animals per group). f–i, n, Lineage-traced, Gli1+ Hh activated lung mesenchymal cells undergo proliferative expansion after repetitive bleomycin injury with an increase in Ki67+ mesenchymal cells (n = 4 animals per group). j–m, o, Expression of SmoM2 within lineage traced Gli1+ lung mesenchymal cells attenuates the proliferative expansion that normally follows repetitive bleomycin injury (n = 3 animals per group). p–v, Gli1 expression remains reduced 2 months after the end of bleomycin treatment (p–r, n = 2 animals per group), which might be due to the persistent scarring that is observed after repetitive bleomycin injury (s–v). AW, airway; V, blood vessel. Scale bars, 100 μm; *P < 0.05. Error bars, mean ± s.d.
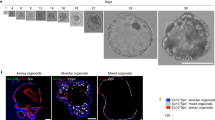
Extended Data Figure 7 Airway epithelium is able to regenerate in vitro and in vivo.
a, b, d, Scgb1a1+ secretory epithelium is initially depleted 3 days following naphthalene injury while TubbIV+ ciliated epithelium remains relatively intact (n = 3 animals per group). c, d, However, 2 months after the initial naphthalene injury, Scgb1a1+ secretory epithelium repopulates the airway and the ratio of secretory/ciliated cells is restored to levels before injury. e, f, GFP+ bronchial epithelial cells isolated from Scgb1a1cre:R26RmTmG animals were cultured in the presence or absence of isolated lung mesenchymal cells, and only those co-cultured with lung mesenchymal cells were able to form organoids. g, h, Examples of the 3-dimensional structures formed by the bronchial organoids in the presence of lung mesenchyme. i–k, Scgb1a1-derived organoids predominantly express markers of secretory airway differentiation, including Scgb1a1 and Nkx2.1 (i, j), while a minority expresses markers of alveolar epithelial lineage including Sftpc (k). l–n, Co-culture of lung mesenchyme and bronchial epithelium induces organoid formation (l), which is inhibited in number (m) and colony size (n) with activation of Hh in the mesenchyme. AW, airway. Scale bars, 100 μm. *P < 0.05. n = 3 animals per group for injury time points. Error bars, mean ± s.d. In vitro organoid studies represent technical quadruplicates, with > 140 randomly selected clones analysed for size per group.
Extended Data Figure 8 Hh signalling mediates both mesenchymal and epithelial quiescence during homeostasis and injury repair in the lung.
The lung epithelium actively maintains mesenchymal quiescence through paracrine Hh signalling, which also regulates a feedback loop to maintain epithelial quiescence. Epithelial injury leads to downregulation of Hh signalling and loss of mesenchymal quiescence, which in turn stimulates epithelial regeneration to replete the airway epithelium until homeostasis is re-established.
Supplementary information
Supplementary Table 1
This table shows GO enrichment analysis for microarray analysis in Extended Data Figure 4. GO analysis was performed on the microarray data obtained from activation of Smo in isolated lung mesenchymal cells. The top 10 (by P value significance) categories identified are shown. (XLSX 9 kb)
Supplementary Table 2
This table contains a list of differentially expressed genes found in the GO category of mitotic nuclear division obtained from activation of Smo in lung mesenchymal cells. (XLSX 23 kb)
Source data
Rights and permissions
About this article
Cite this article
Peng, T., Frank, D., Kadzik, R. et al. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526, 578–582 (2015). https://doi.org/10.1038/nature14984
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14984
This article is cited by
-
Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies
Signal Transduction and Targeted Therapy (2023)
-
Expression of Indian hedgehog signaling in murine oviductal infundibulum and its relationship with epithelial homeostasis
Cell and Tissue Research (2023)
-
Appearing and disappearing acts of cilia
Journal of Biosciences (2023)
-
Abnormal respiratory progenitors in fibrotic lung injury
Stem Cell Research & Therapy (2022)
-
C/EBP homologous protein promotes Sonic Hedgehog secretion from type II alveolar epithelial cells and activates Hedgehog signaling pathway of fibroblast in pulmonary fibrosis
Respiratory Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.