Abstract
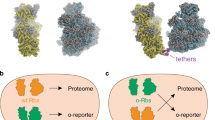
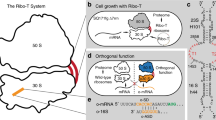
The ribosome is a ribonucleoprotein machine responsible for protein synthesis. In all kingdoms of life it is composed of two subunits, each built on its own ribosomal RNA (rRNA) scaffold. The independent but coordinated functions of the subunits, including their ability to associate at initiation, rotate during elongation, and dissociate after protein release, are an established model of protein synthesis. Furthermore, the bipartite nature of the ribosome is presumed to be essential for biogenesis, since dedicated assembly factors keep immature ribosomal subunits apart and prevent them from translation initiation1. Free exchange of the subunits limits the development of specialized orthogonal genetic systems that could be evolved for novel functions without interfering with native translation. Here we show that ribosomes with tethered and thus inseparable subunits (termed Ribo-T) are capable of successfully carrying out protein synthesis. By engineering a hybrid rRNA composed of both small and large subunit rRNA sequences, we produced a functional ribosome in which the subunits are covalently linked into a single entity by short RNA linkers. Notably, Ribo-T was not only functional in vitro, but was also able to support the growth of Escherichia coli cells even in the absence of wild-type ribosomes. We used Ribo-T to create the first fully orthogonal ribosome–messenger RNA system, and demonstrate its evolvability by selecting otherwise dominantly lethal rRNA mutations in the peptidyl transferase centre that facilitate the translation of a problematic protein sequence. Ribo-T can be used for exploring poorly understood functions of the ribosome, enabling orthogonal genetic systems, and engineering ribosomes with new functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Karbstein, K. Quality control mechanisms during ribosome maturation. Trends Cell Biol. 23, 242–250 (2013)
Hui, A. & de Boer, H. A. Specialized ribosome system: preferential translation of a single mRNA species by a subpopulation of mutated ribosomes in Escherichia coli. Proc. Natl Acad. Sci. USA 84, 4762–4766 (1987)
Rackham, O. & Chin, J. W. A network of orthogonal ribosome·mRNA pairs. Nature Chem. Biol. 1, 159–166 (2005)
Neumann, H., Wang, K., Davis, L., Garcia-Alai, M. & Chin, J. W. Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome. Nature 464, 441–444 (2010)
Erlacher, M. D. et al. Chemical engineering of the peptidyl transferase center reveals an important role of the 2′-hydroxyl group of A2451. Nucleic Acids Res. 33, 1618–1627 (2005)
Kitahara, K. & Suzuki, T. The ordered transcription of RNA domains is not essential for ribosome biogenesis in Escherichia coli. Mol. Cell 34, 760–766 (2009)
Asai, T., Zaporojets, D., Squires, C. & Squires, C. L. An Escherichia coli strain with all chromosomal rRNA operons inactivated: complete exchange of rRNA genes between bacteria. Proc. Natl Acad. Sci. USA 96, 1971–1976 (1999)
Dorywalska, M. et al. Site-specific labeling of the ribosome for single-molecule spectroscopy. Nucleic Acids Res. 33, 182–189 (2005)
Yusupov, M. M. et al. Crystal structure of the ribosome at 5.5 Å resolution. Science 292, 883–896 (2001)
Voorhees, R. M., Weixlbaumer, A., Loakes, D., Kelley, A. C. & Ramakrishnan, V. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nature Struct. Mol. Biol. 16, 528–533 (2009)
Dunkle, J. A. et al. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science 332, 981–984 (2011)
Frank, J. & Agrawal, R. K. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature 406, 318–322 (2000)
Shimizu, Y. et al. Cell-free translation reconstituted with purified components. Nature Biotechnol. 19, 751–755 (2001)
Pédelacq, J. D., Cabantous, S., Tran, T., Terwilliger, T. C. & Waldo, G. S. Engineering and characterization of a superfolder green fluorescent protein. Nature Biotechnol. 24, 79–88 (2006)
Orelle, C. et al. Identifying the targets of aminoacyl-tRNA synthetase inhibitors by primer extension inhibition. Nucleic Acids Res. 41, e144 (2013)
Mankin, A. S. Pactamycin resistance mutations in functional sites of 16S rRNA. J. Mol. Biol. 274, 8–15 (1997)
Orelle, C. et al. Tools for characterizing bacterial protein synthesis inhibitors. Antimicrob. Agents Chemother. 57, 5994–6004 (2013)
Nakatogawa, H. & Ito, K. The ribosomal exit tunnel functions as a discriminating gate. Cell 108, 629–636 (2002)
Vázquez-Laslop, N., Ramu, H., Klepacki, D., Ci, K. & Mankin, A. S. The key role of a conserved and modified rRNA residue in the ribosomal response to the nascent peptide. EMBO J. 29, 3108–3117 (2010)
Bhushan, S. et al. SecM-stalled ribosomes adopt an altered geometry at the peptidyl transferase center. PLoS Biol. 9, e1000581 (2011)
Thompson, J. et al. Analysis of mutations at residues A2451 and G2447 of 23S rRNA in the peptidyltransferase active site of the 50S ribosomal subunit. Proc. Natl Acad. Sci. USA 98, 9002–9007 (2001)
Sato, N. S., Hirabayashi, N., Agmon, I., Yonath, A. & Suzuki, T. Comprehensive genetic selection revealed essential bases in the peptidyl-transferase center. Proc. Natl Acad. Sci. USA 103, 15386–15391 (2006)
Nissen, P., Hansen, J., Ban, N., Moore, P. B. & Steitz, T. A. The structural basis of ribosome activity in peptide bond synthesis. Science 289, 920–930 (2000)
Moll, I., Hirokawa, G., Kiel, M. C., Kaji, A. & Blasi, U. Translation initiation with 70S ribosomes: an alternative pathway for leaderless mRNAs. Nucleic Acids Res. 32, 3354–3363 (2004)
Karamyshev, A. L., Karamysheva, Z. N., Yamami, T., Ito, K. & Nakamura, Y. Transient idling of posttermination ribosomes ready to reinitiate protein synthesis. Biochimie 86, 933–938 (2004)
Cannone, J. J. et al. The Comparative RNA Web (CRW) Site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics 3, 2 (2002)
Arenz, S. et al. Molecular basis for erythromycin-dependent ribosome stalling during translation of the ErmBL leader peptide. Nat. Commun. 5, 3501 (2014)
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods 6, 343–345 (2009)
Fredrick, K., Dunny, G. M. & Noller, H. F. Tagging ribosomal protein S7 allows rapid identification of mutants defective in assembly and function of 30 S subunits. J. Mol. Biol. 298, 379–394 (2000)
Kannan, K., Vázquez-Laslop, N. & Mankin, A. S. Selective protein synthesis by ribosomes with a drug-obstructed exit tunnel. Cell 151, 508–520 (2012)
Ohashi, H., Shimizu, Y., Ying, B. W. & Ueda, T. Efficient protein selection based on ribosome display system with purified components. Biochem. Biophys. Res. Commun. 352, 270–276 (2007)
Merryman, C. & Noller, H. F. in RNA:Protein Interactions, a Practical Approach (ed. Smith, C. W. J. ) 237–253 (Oxford Univ. Press, 1998)
Bundy, B. C. & Swartz, J. R. Site-specific incorporation of p-propargyloxyphenylalanine in a cell-free environment for direct protein-protein click conjugation. Bioconjug. Chem. 21, 255–263 (2010)
Vazquez-Laslop, N., Thum, C. & Mankin, A. S. Molecular mechanism of drug-dependent ribosome stalling. Mol. Cell 30, 190–202 (2008)
An, W. & Chin, J. W. Synthesis of orthogonal transcription-translation networks. Proc. Natl Acad. Sci. USA 106, 8477–8482 (2009)
Inouye, S. & Inouye, M. Up-promoter mutations in the lpp gene of Escherichia coli. Nucleic Acids Res. 13, 3101–3110 (1985)
Schägger, H. & von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379 (1987)
Thomason, L. C., Costantino, N. & Court, D. L. E. coli genome manipulation by P1 transduction. Curr. Prot. Mol. Biol. Chapter 1, Unit–1 17 (2007)
Acknowledgements
We thank I. Ntai for mass spectrometry analysis, K. N. Swonger, C. Burghard, E. M. Fulk, V. Raghavan and N. Aleksashin for help with some experiments, K. Ito for providing the sequence of the pNH122 secM-lacZa reporter, Y. Polikanov for help in preparing ribosome images, J. Lee for assistance in genome sequence analysis, S. Sothiselvam and J. Marks for discussions and suggestions, and N. Vazquez-Laslop for advice on the project and critical reading of the manuscript. This work was supported by the Defense Advanced Research Projects Agency (N66001-12-C-4211), the National Science Foundation grants MCB-0943393 (to M.C.J.) and MCB-1244455 (to A.S.M.) and the David and Lucille Packard Foundation Fellowship (2011-37152) (to M.C.J.).
Author information
Authors and Affiliations
Contributions
M.C.J. and A.S.M. designed the study, analysed results, and wrote the paper. C.O. and E.D.C. designed and performed experiments and analysed data. T.S. and T.F. performed experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Key plasmids used in the study.
a, The pAM552 plasmid is a derivative of pLK35 (ref. 27), from which the unessential segments of the pBR322 cloning vector have been removed. pAM552 contains the entire rrnB operon of E. coli under the control of the phage lambda PL promoter, which is constitutively active in the conventional E. coli strains but is silent at 30 °C in the strain POP2136 (30 °C) carrying the cI857 gene of the temperature-sensitive lambda repressor. The 16S rRNA gene is shown in orange, and the 16S rRNA processing stem sequences indicated in yellow. The 23S rRNA gene is blue, and the corresponding processing stem sequences are light blue. The intergenic tRNAGlu gene is shown in dark grey. b, The map of the pRibo-T8/9 plasmid derived from pAM552. The native 5′ and 3′ ends of the 23S rRNA were linked via a tetranucleotide sequence GAGA (connector C shown in green), and circularly permutated 23 rRNA gene, ‘opened’ in the apex loop of H101, was inserted in the apex loop of 16S rRNA helix h44 via an A8 linker T1 and an A9 linker T2 (red bars). c, The map of the backbone plasmid pT7wtK and the reporter plasmids pT7oGFP and pLpp5oGFP, expressing sf-gfp controlled by an orthogonal Shine–Dalgarno sequence (orange semi-circle) under T7 or lpp5 promoters (black triangles). d, The map of the pACYC177-derived plasmid containing the secM-lacZa reporter gene controlled by the T7 promoter (black triangle) and alternative Shine–Dalgarno sequence (orange semi-circle). The sequence of the secM-lacZa reporter matches that in the originally described plasmid pNH122 (ref. 18).
Extended Data Figure 2 The experimental scheme of preparing and testing circularly permuted 23S rRNA gene library.
a, The CP23S template is generated from pCP23S-EagI plasmid by EagI digestion and ligation. Each CP23S variant is generated by PCR using circularized 23S rRNA gene as a template and a unique primer pair, with added sequences overlapping the destination plasmid backbone. b, The plasmid backbone is prepared by digestion of pAM552-Δ23S-AflII with the AflII restriction enzyme, which linearizes the backbone at the 23S processing stem site. c, Gibson assembly is used to incorporate each CP23S variant into the plasmid backbone to generate the 91 target circular permutants. d, The pAM-CP23S plasmids are transformed into the SQ171 strain lacking chromosomal rRNA operons and carrying the pCSacB plasmid with the wild-type rRNA operon, and transformants resistant to ampicillin, erythromycin and sucrose are selected. e, A complete replacement of pCSacB with pAM-CP23S is verified by a three-primer diagnostic PCR.
Extended Data Figure 3 The Ribo-T tethers allow for the ribosome ratcheting.
Distance changes (Å) between the 16S rRNA and 23S rRNA residues h44 and H101 connected by the oligo(A) linkers in Ribo-T when the ribosome undergoes the transition from the classic to the rotated state. The distances between the 5′ phosphorus atoms of the corresponding nucleotides are shown. 16S and 23S rRNAs in the non-rotated state are tan and pale blue, and in the rotated state are gold and blue, respectively. The structures of the E. coli ribosomes used for measuring the distances and generating the figure have PDB accession numbers 3R8T and 4GD2 (non-rotated state) and 3R8S and 4GD1 (rotated state).
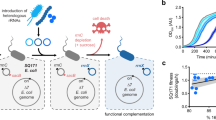
Extended Data Figure 4 Chromosomal mutations enhance growth of SQ171 cells in which Ribo-T completely replace wild-type ribosomes.
a, Growth curves of the parental SQ171 cells transformed with the pAM552(G2058) plasmid (black curve) or pRibo-T8/9 plasmid (blue curve) or selected fast growing mutant (SQ171fg) transformed with pRibo-T8/9 (green curve). The cells express homogeneous populations of ribosomes (wt for pAM552 transformants or Ribo-T for the pRibo-T8/9 transformants, see panels b and c). b, PCR analysis of rDNA in the SQ171fg strain transformed with pRibo-T8/9 (the SQ110 strain that carries a single chromosomal copy of the rrn allele served as a wild-type control). The PCR primers amplify the 302-base-pair 23S rRNA gene segment ‘across’ the H101 hairpin in wild-type rDNA. In pRibo-T, the primer annealing sites are more than 4.8 kb apart (black dashed line), which prevents formation of the PCR product. Two additional primers designed to amplify a 467-bp fragment from the lacZ gene were included in the same PCR reaction as an internal control. The gel is representative of two independent biological experiments. c, Primer extension analysis of rRNA expressed in the SQ171fg cells transformed with pAM552 (WT), pAM552 with the A2058G mutation, or pRibo-T8/9, which carries the A2058G mutation. Primer extension was carried out in the presence of dTTP and ddCTP. Because Ribo-T contains the A2058G mutation in the 23S rRNA sequence, the generated cDNA is one nucleotide shorter than the one generated on the wild-type 23S rRNA template. The lack of the 20-nucleotide cDNA band in the Ribo-T sample demonstrates the absence of wild-type 23S rRNA in the SQ171fg cells transformed with pRibo-T8/9. The gel is representative of three independent biological experiments. d, e, Chromosomal mutations in SQ171fg: a nonsense mutation in the Leu codon 22 of the ybeX gene encoding a protein similar to Mg2+/Co2+ efflux transporter (d); and a missense mutation in codon 549 of the rpsA gene encoding ribosomal protein S1 (e).
Extended Data Figure 5 Ribo-T composition and integrity of the linkers.
a, b, Analysis of rRNA extracted from the isolated wild-type ribosomes or Ribo-T in a denaturing 4% (a) or 8% (b) polyacrylamide gel. a, Ribo-T(1) and Ribo-T(2) represent two individual preparations with Ribo-T(2) isolated following the standard procedure (see Methods), and Ribo-T(1) isolated by immediate pelleting through the sucrose cushion after the cell lysis. The faint bands in the Ribo-T2 preparation indicated by the asterisks could be occasionally seen in some preparations; they probably represent rRNA fragments generated by cleavage of the linkers in a small fraction of Ribo-T either in the cell or during Ribo-T preparation. b, 5S rRNA is present in Ribo-T. c, The relative abundance of small and large subunit proteins in Ribo-T in comparison with wild-type ribosome as determined by mass spectrometry (protein L26 could not be reliably quantified in Ribo-T and wild-type ribosomes). The data represent the average of three technical replicates, and error bars indicate the s.d. d, Analysis of the integrity of the T1 and T2 linkers in a Ribo-T preparation by primer extension. The 22-nucleotide-long primer was extended across the T1 linker in the presence of ddCTP terminator and the 23S-nucleotide-long primer was extended across the T2 linker in the presence of ddGTP terminator. Control samples (−) represent the unextended primers. The gels are representative of two independent experiments.
Extended Data Figure 6 Ribo-T can successfully translate most cellular polypeptides.
a, Protein synthesis rate in SQ171fg cells expressing wild-type ribosomes or Ribo-T. Protein synthesis was measured by quantifying the incorporation of [35S] l-methionine into TCA-insoluble protein fraction during a 45-s incubation at 37 °C in minimal medium. The bar graphs represent the average values of experiments performed in two biological replicates each done in two technical duplicates. Error bars denote s.d. b, c, 2D gel electrophoresis analysis of the proteins expressed in exponentially growing SQ171fg transformed with pAM552 (A2058G) (b) or pRibo-T (c).
Extended Data Figure 7 Chemical probing of the structure of the Ribo-T linkers.
Ribo-T or wild-type ribosomes were modified by dimethylsulfate, and extracted rRNA was subjected to primer extension analysis. In each gel, the left two lanes (‘C’ and ‘A’) represent sequencing reactions followed by dimethylsulfate-modified sample and control (unmodified) RNA. The diagrams on the right represent the secondary structures of helices H101 and h44 in wild-type ribosomes (left) and Ribo-T (right), with the nucleotide residues modified strongly, moderately and weakly indicated by black, grey and white circles, respectively. The gels are representative of two independent experiments.
Extended Data Figure 8 Translation of the orthogonal sf-gfp gene by oRibo-T in vivo and in vitro.
a, Expression of an orthogonal sf-gfp reporter in the E. coli POP2136 cells transformed with pAM552 plasmid encoding wild-type rRNA (wt Rbs), pAM552 with an orthogonal Shine–Dalgarno sequence in 16S rRNA of a non-tethered ribosome (oRbs) or poRibo-T1 expressing an orthogonal Ribo-T (green bar). Cells lacking gfp reporter gene (wt Rbs Δgfp) were used as a background fluorescence control. The data represent the average value of six biological replicates in technical triplicates; error bars indicate the s.d. b, In vitro translation of the orthogonal sf-gfp reporter by non-tethered non-orthogonal wt ribosomes (pink lines), or oRibo-T(A2058G) (which also contained cellular wild-type ribosomes) (green lines). The dotted lines correspond to the translation reactions without antibiotic and solid lines represent reactions supplemented with 50 μM clindamycin (Cld). c, Same as in b, but oRibo-T contained a G693A mutation instead of A2058G and clindamycin was replaced with 100 μM pactamycin (Pct). The red stars indicate the ribosomal subunit carrying the antibiotic-resistance mutation. Graphs in b and c are each representative of two biological replicates each performed in technical triplicates, and error bars indicating the s.d.
Extended Data Figure 9 Promoter mutation in oRibo-T improves transformation of the E. coli cells.
a, b, Several E. coli strains, including BL21 shown in this figure, as well as JM109 and C41, produced slowly growing, heterogeneous colonies when transformed with poRibo-T1. c, Fortuitously, in the course of the experiments we isolated a spontaneous mutant plasmid, poRibo-T2, which showed improved transformation efficiency, producing evenly sized colonies after a single overnight incubation. Sequencing of poRibo-T2 revealed a single mutation in the PL promoter controlling Ribo-T expression, altering the ‘-10’ box from GATACT to TATACT bringing it closer to the TATAAT consensus. It is unclear why the promoter mutation improves performance of poRibo-T (as well as of non-orthogonal pRibo-T) in ‘unselected’ E. coli cells. The plates show representative results of three independent biological experiments.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-2. (PDF 118 kb)
Rights and permissions
About this article
Cite this article
Orelle, C., Carlson, E., Szal, T. et al. Protein synthesis by ribosomes with tethered subunits. Nature 524, 119–124 (2015). https://doi.org/10.1038/nature14862
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14862
This article is cited by
-
The SecM arrest peptide traps a pre-peptide bond formation state of the ribosome
Nature Communications (2024)
-
Resource-aware construct design in mammalian cells
Nature Communications (2023)
-
Community science designed ribosomes with beneficial phenotypes
Nature Communications (2023)
-
Realization of Arithmetic Operations using a Combined Computational Unit in Ribosomal Computing
Journal of The Institution of Engineers (India): Series B (2023)
-
Cell-free Biosynthesis of Peptidomimetics
Biotechnology and Bioprocess Engineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.