Abstract
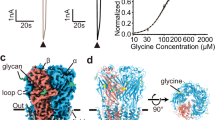
The strychnine-sensitive glycine receptor (GlyR) mediates inhibitory synaptic transmission in the spinal cord and brainstem and is linked to neurological disorders, including autism and hyperekplexia. Understanding of molecular mechanisms and pharmacology of glycine receptors has been hindered by a lack of high-resolution structures. Here we report electron cryo-microscopy structures of the zebrafish α1 GlyR with strychnine, glycine, or glycine and ivermectin (glycine/ivermectin). Strychnine arrests the receptor in an antagonist-bound closed ion channel state, glycine stabilizes the receptor in an agonist-bound open channel state, and the glycine/ivermectin complex adopts a potentially desensitized or partially open state. Relative to the glycine-bound state, strychnine expands the agonist-binding pocket via outward movement of the C loop, promotes rearrangement of the extracellular and transmembrane domain ‘wrist’ interface, and leads to rotation of the transmembrane domain towards the pore axis, occluding the ion conduction pathway. These structures illuminate the GlyR mechanism and define a rubric to interpret structures of Cys-loop receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Primary accessions
Electron Microscopy Data Bank
Protein Data Bank
Data deposits
Three three-dimensional cryo-EM density maps and coordinates of α1 glycine receptors in strychnine-bound, glycine-bound and glycine/ivermectin-bound forms have been deposited in the Electron Microscopy Data Bank under the accession numbers EMD-6344, EMD-6345, and EMD-6346 and deposited in the RCSB Protein Data Bank under the accession codes 3JAD, 3JAE, and 3JAF.
References
Katz, B. & Thesleff, S. A study of the ‘desensitization’ produced by acetylcholine at the motor end-plate. J. Physiol. (Lond.) 138, 63–80 (1957)
Aprison, M. H. & Werman, R. The distribution of glycine in cat spinal cord and roots. Life Sci. 4, 2075–2083 (1965)
Curtis, D. R., Hosli, L. & Johnston, G. A. Inhibition of spinal neurons by glycine. Nature 215, 1502–1503 (1967)
Werman, R., Davidoff, R. A. & Aprison, M. H. Inhibition of motoneurones by iontophoresis of glycine. Nature 214, 681–683 (1967)
Curtis, D. R., Hosli, L., Johnston, G. A. & Johnston, I. H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp. Brain Res. 5, 235–258 (1968)
Lynch, J. W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 84, 1051–1095 (2004)
Galzi, J. L., Edelstein, S. J. & Changeux, J. The multiple phenotypes of allosteric receptor mutants. Proc. Natl Acad. Sci. USA 93, 1853–1858 (1996)
Legendre, P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 58, 760–793 (2001)
Lynch, J. W. & Callister, R. J. Glycine receptors: a new therapeutic target in pain pathways. Curr. Opin. Investig. Drugs 7, 48–53 (2006)
Bode, A. & Lynch, J. W. The impact of human hyperekplexia mutations on glycine receptor structure and function. Mol. Brain 7, 2 (2014)
Young, A. B. & Snyder, S. H. Strychnine binding associated with glycine receptors of the central nervous system. Proc. Natl Acad. Sci. USA 70, 2832–2836 (1973)
Pfeiffer, F., Graham, D. & Betz, H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J. Biol. Chem. 257, 9389–9393 (1982)
Graham, D., Pfeiffer, F. & Betz, H. Photoaffinity-labelling of the glycine receptor of rat spinal cord. Eur. J. Biochem. 131, 519–525 (1983)
Bormann, J., Hamill, O. P. & Sakmann, B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. 385, 243–286 (1987)
Fatima-Shad, K. & Barry, P. H. Anion permeation in GABA- and glycine-gated channels of mammalian cultured hippocampal neurons. Proc. R. Soc. Lond. B 253, 69–75 (1993)
Shan, Q., Haddrill, J. L. & Lynch, J. W. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J. Biol. Chem. 276, 12556–12564 (2001)
Changeux, J. P. & Edelstein, S. J. Allosteric receptors after 30 years. Neuron 21, 959–980 (1998)
Miller, P. S. & Smart, T. G. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol. Sci. 31, 161–174 (2010)
Bocquet, N. et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114 (2009)
Hilf, R. J. & Dutzler, R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118 (2009)
Spurny, R. et al. Pentameric ligand-gated ion channel ELIC is activated by GABA and modulated by benzodiazepines. Proc. Natl Acad. Sci. USA 109, E3028–E3034 (2012)
Sauguet, L. et al. Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation. Proc. Natl Acad. Sci. USA 111, 966–971 (2014)
Moraga-Cid, G. et al. Allosteric and hyperekplexic mutant phenotypes investigated on an α1 glycine receptor transmembrane structure. Proc. Natl Acad. Sci. USA 112, 2865–2870 (2015)
Miyazawa, A., Fujiyoshi, Y. & Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949–955 (2003)
Unwin, N. Refined structure of the nicotinic acetylcholine receptor. J. Mol. Biol. 346, 967–989 (2005)
Hibbs, R. E. & Gouaux, E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60 (2011)
Althoff, T., Hibbs, R. E., Banerjee, S. & Gouaux, E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature 512, 333–337 (2014)
Hassaine, G. et al. X-ray structure of the mouse serotonin 5–HT3 receptor. Nature 512, 276–281 (2014)
Miller, P. S. & Aricescu, A. R. Crystal structure of a human GABAA receptor. Nature 512, 270–275 (2014)
daCosta, C. J. & Baenziger, J. E. Gating of pentameric ligand-gated ion channels: structural insights and ambiguities. Structure 21, 1271–1283 (2013)
Cecchini, M. & Changeux, J. P. The nicotinic acetylcholine receptor and its prokaryotic homologues: structure, conformational transitions & allosteric modulation. Neuropharmacology 96, 137–149 (2015)
Hille, B. Ion Channels of Excitable Membranes (Sinauer Associates, 2001)
Kinde, M. N. et al. Conformational changes underlying desensitization of the pentameric ligand-gated ion channel ELIC. Structure 23, 995–1004 (2015)
Gielen, M., Thomas, P. & Smart, T. G. The desensitization gate of inhibitory Cys-loop receptors. Nature Commun. 6, 6829 (2015)
Akabas, M. H. Using molecular dynamics to elucidate the structural basis for function in pLGICs. Proc. Natl Acad. Sci. USA 110, 16700–16701 (2013)
Brams, M. et al. A structural and mutagenic blueprint for molecular recognition of strychnine and d-tubocurarine by different cys-loop receptors. PLoS Biol. 9, e1001034 (2011)
Vandenberg, R. J., Handford, C. A. & Schofield, P. R. Distinct agonist- and antagonist-binding sites on the glycine receptor. Neuron 9, 491–496 (1992)
Marvizón, J. C. et al. The glycine receptor: pharmacological studies and mathematical modeling of the allosteric interaction between the glycine- and strychnine-binding sites. Mol. Pharmacol. 30, 590–597 (1986)
Ruiz-Gómez, A., Morato, E., Garcia-Calvo, M., Valdivieso, F. & Mayor, F., Jr Localization of the strychnine binding site on the 48-kilodalton subunit of the glycine receptor. Biochemistry 29, 7033–7040 (1990)
Rajendra, S. & Schofield, P. R. Molecular mechanisms of inherited startle syndromes. Trends Neurosci. 18, 80–82 (1995)
Rajendra, S. et al. The unique extracellular disulfide loop of the glycine receptor is a principal ligand binding element. EMBO J. 14, 2987–2998 (1995)
Yu, R. et al. Agonist and antagonist binding in human glycine receptors. Biochemistry 53, 6041–6051 (2014)
Grudzinska, J. et al. The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 45, 727–739 (2005)
Mukhtasimova, N., Free, C. & Sine, S. M. Initial coupling of binding to gating mediated by conserved residues in the muscle nicotinic receptor. J. Gen. Physiol. 126, 23–39 (2005)
Hansen, S. B. et al. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 24, 3635–3646 (2005)
Purohit, P. & Auerbach, A. Loop C and the mechanism of acetylcholine receptor-channel gating. J. Gen. Physiol. 141, 467–478 (2013)
Celie, P. H. et al. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41, 907–914 (2004)
Huang, S. et al. Complex between α-bungarotoxin and an α7 nicotinic receptor ligand-binding domain chimaera. Biochem. J. 454, 303–310 (2013)
Lynagh, T. & Lynch, J. W. An improved ivermectin-activated chloride channel receptor for inhibiting electrical activity in defined neuronal populations. J. Biol. Chem. 285, 14890–14897 (2010)
Purohit, P., Gupta, S., Jadey, S. & Auerbach, A. Functional anatomy of an allosteric protein. Nature Commun. 4, 2984 (2013)
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010)
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nature Methods 10, 584–590 (2013)
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012)
Mindell, J. A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003)
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007)
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nature Methods 9, 853–854 (2012)
Arnold, K., Bordoli, L., Kopp, J. & Schwede, T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006)
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011)
Amunts, A. et al. Structure of the yeast mitochondrial large ribosomal subunit. Science 343, 1485–1489 (2014)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
DeLano, W. L. The PyMOL Molecular Graphics System. (DeLano Scientific, San Carlos, USA, 2002)
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360 (1996)
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nature Methods 11, 63–65 (2014)
Acknowledgements
We are grateful to Z. H. Yu, N. Grigorieff, J. Cruz and C. Hong (Janelia Campus), C. Arthur (FEI) and M. Braunfeld (UCSF) for assistance with microscope operation, data collection and for comments, and to R. Stites, M. Hakanson and A. Trzynka (OHSU) for computational support. We acknowledge the support of R. Goodman and J. Gray. Microscopy at Oregon Health & Science University (OHSU) was performed at the Multiscale Microscopy Core (MMC) with technical support from the OHSU-FEI Living Lab, Intel and the OHSU Center for Spatial Systems Biomedicine (OCSSB). We thank L. Vaskalis for help with illustrations and H. Owen for proofreading. R. Hibbs is gratefully acknowledged for pre-screening the GlyR constructs and D. P. Claxton for optimizing the constructs. We thank Gouaux and Baconguis laboratory members for discussions. This work was supported by the National Institute of Health (E.G). E.G. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
J.D., W.L. and E.G. designed the project, J.D. and W.L. performed sample preparation, cryo-EM data collection and data analysis, J.D., W.L. and E.G. wrote the manuscript, S.W. and Y.C. assisted in cryo-EM experiments at UCSF and participated in discussion and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
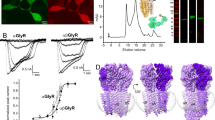
Extended Data Figure 1 Three-dimensional reconstruction of strychnine-bound GlyR.
a, A representative micrograph (out of 1,829 micrographs) of strychnine-bound GlyR in vitreous ice. b, c, Angular distribution of particle projections (b), and selected 2D classes (c) are shown. In c, the radius of the sphere is proportional to the number of particles assigned to it. The plot is drawn with respect to the 3D reconstruction shown in the centre, taking the C5 symmetry of the receptor into account. d, Selected ‘slice’ views of the final reconstruction along the pore axis. The slice numbers are indicated, starting from the intracellular side. e, FSC curves for the density maps before (red) and after (black) post-processing in RELION. The FSC curve between the refined atomic model and the final reconstruction map is shown in green. f, FSC curves for cross-validation: model versus summed map (full data set, green), model versus half map 1 (used in test refinement, orange) and model versus half map 2 (not used in test refinement, purple). g, Unfiltered and unsharpened 3D density map coloured according to local resolution estimated using RESMAP65. h, Real-space correlation between atomic model and density map calculated using PHENIX.
Extended Data Figure 2 3D reconstruction of glycine-bound GlyR.
a, A representative micrograph (out of 1,460 migrographs) of glycine-bound GlyR in vitreous ice. b, c, Angular distribution of particle projections (b) and selected 2D classes (c) are shown. In c, the radius of the sphere is proportional to the number of particles assigned to it. The plot is drawn with respect to the 3D reconstruction shown in the centre, taking the C5 symmetry of the receptor into account. d, Selected ‘slice’ views of the final reconstruction along the pore axis. The slice numbers are indicated, starting from the intracellular side. e, FSC curves for the density maps before (red) and after (black) post-processing in RELION. The FSC curve between the refined atomic model and the final reconstruction map is shown in green. f, FSC curves for cross-validation: model versus summed map (full data set, green), model versus half map 1 (used in test refinement, orange) and model versus half map 2 (not used in test refinement, purple). g, Unfiltered and unsharpened 3D density map coloured according to local resolution estimated using RESMAP. h, Real-space correlation between atomic model and density map calculated using PHENIX.
Extended Data Figure 3 3D reconstruction of glycine/ivermectin-bound GlyR.
a, A representative micrograph (out of 2,489 micrographs) of glycine/ivermectin-bound GlyR in vitreous ice. b, c, Angular distribution of particle projections (b) and selected 2D classes (c) are shown. c, The radius of the sphere is proportional to the number of particles assigned to it. The plot is drawn with respect to the 3D reconstruction shown in the centre, taking the C5 symmetry of the receptor into account. d, Selected ‘slice’ views of the final reconstruction along the pore axis. The slice numbers are indicated, starting from the intracellular side. e, FSC curves for the density maps before (red) and after (black) post-processing in RELION. The FSC curve between the refined atomic model and the final reconstruction map is shown in green. f, FSC curves for cross-validation: model versus summed map (full data set, green), model versus half map 1 (used in test refinement, orange) and model versus half map 2 (not used in test refinement, purple). g, Unfiltered and unsharpened 3D density map coloured according to local resolution estimated using RESMAP. h, Real-space correlation between atomic model and density map calculated using PHENIX.
Extended Data Figure 4 Representative densities of the three reconstructions of GlyR. Densities are sharpened using RELION unless indicated otherwise.
The densities in each panel are for the strychnine-, glycine/ivermectin-, glycine-, and unsharpened glycine-bound states, respectively, from left to right. a, Representative densities of the β-sheets in ECD, contoured at 8σ. b, Densities of Cys loop and the M2–M3 loop, contoured at 7σ. c, Densities of helices M1 and M2, contoured at 7σ. d, Densities of M3 and M4, contoured at 7σ. e, Densities of −2′Pro, contoured at 7σ except for the glycine-bound state (6.5σ). f, Densities of 9′Leu, contoured at 6.0σ except for the glycine-bound state (5.0σ).
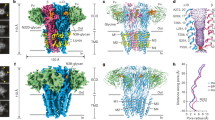
Extended Data Figure 5 A single subunit of glycine/ivermectin bound GlyR.
a–c, Viewed in parallel to the membrane plane, with secondary structure elements labelled. b, The domain arrangement resembles an upright forearm, clad with a mitten, consisting of thumb (C loop), palm (β-strands of ECD) and ligament (ECD–TMD interface).
Extended Data Figure 6 Comparison of ivermectin-binding site in GlyR (red) and GluCl (green).
a, Viewed in parallel to the membrane. b, Viewed from the extracellular side. The (+)-subunits are shown in darker colours. The residue corresponding to Arg287, which forms a hydrogen bond with the ivermectin in GlyR, is an asparagine (Asn264) in GluCl. The corresponding residue of Val296 in the M2–M3 loop of GlyR is an isoleucine (Ile273) in GluCl, whose larger side chain prevents the upper tip of ivermectin from approaching and interacting with the main chain oxygen atom of Ser721 in the M2–M3 loop (Ser294 in GlyR). The Gly237 in the M1 and Ala304 in the M3 of GlyR are Ser217 and Gly281 in GluCl, respectively. Such differences on side chains weaken or strengthen the interaction of ivermectin with M3 or M1 in GlyR, respectively, in comparison to that in GluCl.
Extended Data Figure 7 Comparison of GlyR with other Cys-loop receptors.
a, The two restriction sites, viewed from the cytoplasmic side. The Cα of −2′Pro equivalents (cyan) and 9′Leu equivalents (magenta) are shown as spheres. Distances between adjacent Cα atoms are labelled. b, Plot of the vector connecting the −2′ProCα equivalent and 9′LeuCα equivalent, with −2ProCα equivalent as the origin, the tilt angle θ and the rotation angle ϕ relative to the pore axis. The ϕ of strychnine-bound GlyR is arbitrarily set to zero. c, Pore radii as a function of distance along the pore axis, calculated using the program HOLE, where the Cα position of 0′Arg is set to zero. d, Table showing parameters of the vector connecting the −2′ProCα equivalent and 9′LeuCα equivalent, where r is the distance from −2′ProCα equivalent to 9′LeuCα equivalent, RestrP and RestrL are the pore radii at −2′Pro equivalent and 9′Leu equivalent, respectively. The dC loop is the distance between Cα of Thr220 equivalent and Leu143 equivalent, representing the opening of the C loop shown in e. e, Comparison of ligand-binding pockets. The side chains of marker residues are shown in stick format.
Extended Data Figure 8 Superimposition of TMD in three GlyR structures using main chain atoms of residues Met236–Lys362.
a, Between strychnine- and glycine–GlyR. b, Strychnine- and glycine/ivermectin–GlyR. c, Glycine- and glycine/ivermectin–GlyR. The M2–M3 loop, residues Ser289–Ala298, is excluded from the comparison. The root mean square deviations (r.m.s.d.) are 0.9, 0.9, and 0.7 Å, respectively, suggesting that the movement of the TMD is rigid-body-like. Most differences are located in the termini of transmembrane helices, which are either close to the M2–M3 loop, or close to the intracellular gate −2′Pro.
Extended Data Figure 9 Positions of residues in which mutations are associated with human startle disease.
Residues that likely interact with disease‐causing residues are labelled in italics. a, The strychnine–GlyR model is used to show residues in which mutations cause spontaneous activation. The mutation of Gln242 in M1 to glutamate may enhance its electrostatic attraction to Arg287 in M2 of the adjacent subunit and tilt the upper part of M2 away from pore axis, resulting in a constitutively open channel. Alternatively, the mutation Val296Met in M2–M3 loop may cause steric collision with Ile241 in M1 of the adjacent subunit, and prevent Ser294 from interacting to the N‐cap formed by pre‐M1, M1 and the β8–β9 loop, thereby destabilizing the closed conformation. b, The glycine/ivermectin–GlyR model is used to show residues in the ECD–TMD interface whose mutations reduce sensitivity to glycine and single channel conductance. The mutation of Arg234 in pre‐M1 to glutamine may disturb its electrostatic interaction with Asp164 in the Cys loop. Similarly, the mutation of Tyr295 in the M2–M3 loop to cysteine or serine may disturb its interaction with the main chain nitrogen atom of Leu158 in the Cys loop. In both cases, the signal induced by agonist binding may be blocked. The mutation Lys292Glu in the M2–M3 loop possibly affects the cooperative interaction between two adjacent subunits by altering the van der Waals contacts between Lys292 and Tyr238. c, The glycine–GlyR model is used to show residues in M2 in which mutations reduce sensitivity to glycine and diminish single channel conductance. These mutations may directly influence the pore properties by modifying the interactions with adjacent residues, for instance, between Gln282His and Pro246, and between Arg287Gln/Leu and Gln242.
Supplementary information
Supplementary Figures
This file contains Supplementary Figure 1. (PDF 140 kb)
Ligand-induced conformational transitions of GlyR
This video shows a morph of the GlyR from the strychnine-bound to the glycine/ivermectin-bound state, via the glycine-bound state. Shown on the left side of the video is the view from the cytoplasmic side of the membrane and on the right side is the view parallel to the membrane. One subunit is highlighted. (AVI 11206 kb)
Rights and permissions
About this article
Cite this article
Du, J., Lü, W., Wu, S. et al. Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 526, 224–229 (2015). https://doi.org/10.1038/nature14853
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14853
This article is cited by
-
Patch-clamp studies and cell viability assays suggest a distinct site for viroporin inhibitors on the E protein of SARS-CoV-2
Virology Journal (2023)
-
Neuroprotection elicited by taurine in sporadic Alzheimer-like disease: benefits on memory and control of neuroinflammation in the hippocampus of rats
Molecular and Cellular Biochemistry (2023)
-
Anti-seizure properties of Ipomoea asarifolia (Desr.) (Convolvulaceae) ethanolic leaf extract in laboratory animals
Bulletin of the National Research Centre (2022)
-
Structural basis for cannabinoid-induced potentiation of alpha1-glycine receptors in lipid nanodiscs
Nature Communications (2022)
-
Fluorescence-detection size-exclusion chromatography utilizing nanobody technology for expression screening of membrane proteins
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.