Abstract
Stress is considered a potent environmental risk factor for many behavioural abnormalities, including anxiety and mood disorders1,2. Animal models can exhibit limited but quantifiable behavioural impairments resulting from chronic stress, including deficits in motivation, abnormal responses to behavioural challenges, and anhedonia3,4,5. The hippocampus is thought to negatively regulate the stress response and to mediate various cognitive and mnemonic aspects of stress-induced impairments2,3,5, although the neuronal underpinnings sufficient to support behavioural improvements are largely unknown. Here we acutely rescue stress-induced depression-related behaviours in mice by optogenetically reactivating dentate gyrus cells that were previously active during a positive experience. A brain-wide histological investigation, coupled with pharmacological and projection-specific optogenetic blockade experiments, identified glutamatergic activity in the hippocampus–amygdala–nucleus-accumbens pathway as a candidate circuit supporting the acute rescue. Finally, chronically reactivating hippocampal cells associated with a positive memory resulted in the rescue of stress-induced behavioural impairments and neurogenesis at time points beyond the light stimulation. Together, our data suggest that activating positive memories artificially is sufficient to suppress depression-like behaviours and point to dentate gyrus engram cells as potential therapeutic nodes for intervening with maladaptive behavioural states.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Caspi, A. et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389 (2003)
Pittenger, C. & Duman, R. S. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33, 88–109 (2008)
Hyman, S. E. Revitalizing psychiatric therapeutics. Neuropsychopharmacology 39, 220–229 (2014)
Covington, H. E., III et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 30, 16082–16090 (2010)
Russo, S. J. & Nestler, E. J. The brain reward circuitry in mood disorders. Nature Rev. Neurosci. 14, 609–625 (2013)
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012)
Ramirez, S. et al. Creating a false memory in the hippocampus. Science 341, 387–391 (2013)
Redondo, R. L. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014)
Seligman, M. E. P., Rashid, T. & Parks, A. C. Positive psychotherapy. Am. Psychol. 61, 774–788 (2006)
Tye, K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013)
Warden, M. R. et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492, 428–432 (2012)
Deisseroth, K. Circuit dynamics of adaptive and maladaptive behaviour. Nature 505, 309–317 (2014)
Lim, B. K., Huang, K. W., Grueter, B. A., Rothwell, P. E. & Malenka, R. C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 (2012)
Snyder, J. S., Soumier, A., Brewer, M., Pickel, J. & Cameron, H. A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461 (2011)
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012)
Dölen, G., Darvishzadeh, A., Huang, K. W. & Malenka, R. C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013)
Schlaepfer, T. E. et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33, 368–377 (2008)
Xiu, J. et al. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nature Neurosci. 17, 1552–1559 (2014)
Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011)
DeRubeis, R. J., Siegle, G. J. & Hollon, S. D. Cognitive therapy versis medication. Nature Rev. Neurosci. 9, 788–796 (2008)
Han, X. et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front. Syst. Neurosci. 5, 18 (2011)
Brody, A. L. et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy. Arch. Gen. Psychiatry 58, 631–640 (2001)
Airan, R. D. et al. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317, 819–823 (2007)
Seki, T. & Arai, Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J. Neurosci. 13, 2351–2358 (1993)
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003)
Roozendaal, B., McEwen, B. S. & Chattarji, S. Stress, memory and the amygdala. Nature Rev. Neurosci. 10, 423–433 (2009)
Felix-Ortiz, A. C. et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664 (2013)
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354 (2000)
Friedman, A. K. et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344, 313–319 (2014)
Tsankova, N., Renthal, W., Kumar, A. & Nestler, E. J. Epigenetic regulation in psychiatric disorders. Nature Rev. Neurosci. 8, 355–367 (2007)
Reijmers, L. G., Perkins, B. L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007)
Redondo, R. L. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014)
Ramirez, S. et al. Creating a false memory in the hippocampus. Science 341, 387–391 (2013)
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012)
Han, X. et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front. Syst. Neurosci. 5, 18 (2011)
Huff, M. L., Miller, R. L., Deisseroth, K., Moorman, D. E. & LaLumiere, R. T. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc. Natl Acad. Sci. USA 110, 3597–3602 (2013)
Snyder, J. S., Soumier, A., Brewer, M., Pickel, J. & Cameron, H. A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461 (2011)
Acknowledgements
We thank B. Chen, D. S. Roy, and J. Kim for help with the experiments, T. J. Ryan and T. Kitamura for the TRE–ArchT–eGFP construct, J. Sarinana and E. Hueske for comments and extensive discussions on the manuscript, and all the members of the Tonegawa laboratory for their support. We dedicate this study to the memory of Xu Liu, who made major contributions to memory engram research. This work was supported by RIKEN Brain Science Institute and Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
S.R., X.L., C.M., A.M., J.Z., R.L.R. and S.T. contributed to the study design. S.R., X.L., A.M., J.Z., C.M. and R.L.R. contributed to the data collection and interpretation. X.L. cloned all constructs. S.R., X.L., C.M., J.Z. and A.M. conducted the surgeries, behaviour experiments, and histological analyses. S.R., X.L. and S.T. wrote the paper. All authors discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
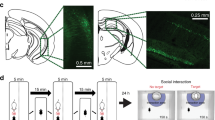
Extended Data Figure 1 Male mice spend more time around an object associated with females.
Top, Time spent in the target zone where the object associated with females is introduced in the ON phases. Female-object paired mice (experimental group) spend more time in the target zone during the ON phases than the neutral-object paired mice (control group; two-way ANOVA with multiple comparisons, ON1 t88 = 2.41; P < 0.05, ON2 t88 = 2.08; P < 0.05). Bottom, Difference score (average of ON phases − baseline (Bsl)) also shows the increased preference for the target zone in the female-object group compared to neutral-object group (t22 = 2.37; *P < 0.05). n = 12 per group. See the Methods section for detailed methods.
Extended Data Figure 2 Positive, neutral, or negative experiences label a similar proportion of dentate gyrus cells with ChR2; stress prevents weight gain over 10 days.
a, The c-Fos mice were bilaterally injected with AAV9-TRE-ChR2-mCherry and implanted with optical fibres targeting the dentate gyrus. b–e, Histological quantifications reveal that, while off Dox, a similar proportion of dentate gyrus cells are labelled by ChR2–mCherry in response to a positive (c), neutral (d), or negative (e) experience. All animals were sacrificed a day after completing the CIS protocol. One-way ANOVA followed by Bonferroni post hoc test, P > 0.05, n.s., not significant. f, Animals were chronically immobilized for 10 days, during which they lost a significant amount of weight compared to an unstressed group (one-way ANOVA followed by Bonferroni post hoc test, *P < 0.05, n = 9 per group). Data are means ± s.e.m.
Extended Data Figure 3 Reactivation of a positive memory decreases latency to feed in a novelty suppressed feeding paradigm.
a, All groups were food deprived for 24 h and then underwent a novelty-suppressed feeding protocol. While chronic immobilization increased the latency to feed, light-reactivation of a positive memory significantly decreased the latency to feed at levels that matched the unstressed groups. b, Upon completion of the novelty suppressed feeding test, all groups were returned to their home cage and food intake was measured after 5 min (one-way ANOVA followed by Bonferroni post hoc test, **P < 0.01, n = 16 per group). Data are means ± s.e.m.
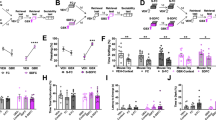
Extended Data Figure 4 Activation of a positive memory elicits BLA spiking activity, requires NAcc glutamatergic activity in the tail suspension test, but does not alter locomotor activity in the open field test.
a, Raster plots and peri-stimulus time histograms (PSTH) illustrating a transient excitatory response from a single BLA neuron out of the nine neurons responsive to dentate gyrus positive memory activation during 10 s of blue light stimulation, but not in response to 10 s of red light as a control. Blue bar plots on the right illustrate maximum BLA neural firing rate before (Pre) and after (Post) blue light stimulation in the dentate gyrus (paired t-test, t7 = 6.91, *P = 0.023). Red bar plots show the maximum neural activity for the same neurons after red light stimulation in the dentate gyrus that serves as a control (paired t-test, t7 = 1.62, P = 0.15). b, Brain diagram illustrating target areas manipulated. Within-subjects experiments revealed that glutamatergic antagonists (Glux), but not saline, in the accumbens shell blocked the light-induced effects of a positive memory in stressed subjects. For behavioural data, a two-way ANOVA with repeated measures followed by a Bonferroni post hoc test revealed a group-by-light epoch interaction on day 1 (F1,90 = 28.39, P < 0.001; n = 16 per group) and day 2 of testing (F1,90 = 8.28, P < 0.01). Data are means ± s.e.m. c, All groups failed to show significant changes in locomotor activity within a session of open field exploration during either light off or light on epochs, though any trends towards decreases in locomotion are consistent with stress-induced behavioural impairments. *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Figure 5 Activating a positive memory in the dentate gyrus produces an increase in c-Fos expression in the lateral septum and hypothalamus, but not the lateral habenula, ventral hippocampus, or VTA.
a, Diagram of regions analysed. b, c, c-Fos expression significantly increased in the lateral septum (b) and subregions of the hypothalamus including the dorsomedial (DM), ventromedial (VM), and lateral hypothalamus (c) in the positive memory group but not in a group in which a neutral memory was stimulated or in a group expressing mCherry alone. c–e, c-Fos expression did not significantly increase in the lateral habenula (c), various ventral hippocampus subregions (d), or VTA, identified by tyrosine hydroxylase staining (red) stainings in the images expanded on the right (e) (one-way ANOVA followed by Bonferroni post hoc test *P < 0.05, **P < 0.01, n = 5 animals per group, 3–5 slices per animal). TS, tail suspension. Data are means ± s.e.m.
Extended Data Figure 6 Activating a positive memory through the dentate gyrus of unstressed animals increases c-Fos expression in various downstream regions.
a, Diagram of regions analysed. b, In the positive compared to the neutral memory group, c-Fos expression is significantly increased in the lateral septum, NAcc shell, BLA, dorsomedial, ventromedial and lateral hypothalamus, but not in the mPFC, NAcc core, habenula, or ventral hippocampus. Trends were observed in the central amygdala (CeA) and VTA. Each brain region was analysed using an unpaired Student’s t test, n = 5 animals per group, 3–5 slices per animal; #P = 0.17 for central amygdala and P = 0.09 for VTA; *P < 0.05, **P < 0.01, ***P < 0.001, n.s., not significant. Data are means ± s.e.m.
Extended Data Figure 7 Dopamine receptor antagonists block the light-induced effects of positive memory activation; a single session of activating a positive memory in the dentate gyrus does not produce long-lasting antidepressant-like effects.
a, Administration of a cocktail of dopamine receptor antagonists (DAx) prevented the light-induced increases in struggling during the tail suspension test. When animals were tested again on day 2 and infused with saline, the behavioural effects of optically reactivating a positive memory were observed (two-way ANOVA with repeated measures followed by Bonferroni post hoc test, *P < 0.05, n = 9 per group). b, Animals in which a positive memory was optically activated during the tail suspension test or sucrose preference test showed acute increases in time struggling or preference for sucrose; this change in behaviour did not persist when tested again on day 2 (within subjects ANOVA followed by Bonferroni post hoc test), n = 9. n.s., not significant. Data are means ± s.e.m.
Extended Data Figure 8 Chronic activation of a positive memory prevents stress-induced decreases in neurogenesis.
a, The 5-day positive memory stimulation group showed a significant increase of adult newborn cells in the dentate gyrus as measured by doublecortin (DCX)-positive cells (one-way ANOVA followed by Bonferroni post hoc test, F5,72 = 7.634, P < 0.01) relative to control groups. b–g, Representative images of DCX-positive cells in the dentate gyrus for the 5-day (b), 1-day (c), neutral (d), no stimulation (e), natural (f), and no stress (g) groups. h–m, Representative PSA-NCAM images corresponding to data appearing in Fig. 4d. n = 5 slices per animal, 13 animals per group for data appearing in a. *P < 0.05, n.s., not significant. Data are means ± s.e.m.
Extended Data Figure 9 Behavioural and neuronal correlations.
a, Performance levels in the SPT and the number of adult-born neurons as measured by PSA-NCAM are positively correlated on an animal-by-animal basis. b, Performance levels between the TST and SPT show strong positive correlation trends on an animal-by-animal basis. n = 14 per TST behavioural group, n = 15 per SPT behavioural group.
Rights and permissions
About this article
Cite this article
Ramirez, S., Liu, X., MacDonald, C. et al. Activating positive memory engrams suppresses depression-like behaviour. Nature 522, 335–339 (2015). https://doi.org/10.1038/nature14514
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14514
This article is cited by
-
Formation and fate of an engram in the lateral amygdala supporting a rewarding memory in mice
Neuropsychopharmacology (2023)
-
A computational analysis of mouse behavior in the sucrose preference test
Nature Communications (2023)
-
ASMT determines gut microbiota and increases neurobehavioral adaptability to exercise in female mice
Communications Biology (2023)
-
Assessments of dentate gyrus function: discoveries and debates
Nature Reviews Neuroscience (2023)
-
Brain-wide projection reconstruction of single functionally defined neurons
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.