Abstract
One of the characteristics of the central nervous system is the lack of a classical lymphatic drainage system. Although it is now accepted that the central nervous system undergoes constant immune surveillance that takes place within the meningeal compartment1,2,3, the mechanisms governing the entrance and exit of immune cells from the central nervous system remain poorly understood4,5,6. In searching for T-cell gateways into and out of the meninges, we discovered functional lymphatic vessels lining the dural sinuses. These structures express all of the molecular hallmarks of lymphatic endothelial cells, are able to carry both fluid and immune cells from the cerebrospinal fluid, and are connected to the deep cervical lymph nodes. The unique location of these vessels may have impeded their discovery to date, thereby contributing to the long-held concept of the absence of lymphatic vasculature in the central nervous system. The discovery of the central nervous system lymphatic system may call for a reassessment of basic assumptions in neuroimmunology and sheds new light on the aetiology of neuroinflammatory and neurodegenerative diseases associated with immune system dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
24 February 2016
A Correction to this paper has been published: https://doi.org/10.1038/nature16999
References
Ransohoff, R. M. & Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nature Rev. Immunol. 12, 623–635 (2012).
Kipnis, J., Gadani, S. & Derecki, N. C. Pro-cognitive properties of T cells. Nature Rev. Immunol. 12, 663–669 (2012).
Shechter, R., London, A. & Schwartz, M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nature Rev. Immunol. 13, 206–218 (2013).
Goldmann, J. et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J. Leukoc. Biol. 80, 797–801 (2006).
Kaminski, M. et al. Migration of monocytes after intracerebral injection at entorhinal cortex lesion site. J. Leukoc. Biol. 92, 31–39 (2012).
Engelhardt, B. & Ransohoff, R. M. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 26, 485–495 (2005).
Alitalo, K. The lymphatic vasculature in disease. Nature Med. 17, 1371–1380 (2011).
Wang, Y. & Oliver, G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 24, 2115–2126 (2010).
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007).
Kerjaschki, D. The lymphatic vasculature revisited. J. Clin. Invest. 124, 874–877 (2014).
Debes, G. F. et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nature Immunol. 6, 889–894 (2005).
Weber, M. et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339, 328–332 (2013).
Bazigou, E. et al. Integrin-α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell 17, 175–186 (2009).
Koina, M. E. et al. Evidence for lymphatics in the developing and adult human choroid. Invest. Ophthalmol. Vis. Sci. 56, 1310–1327 (2015).
Johanson, C. E. et al. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 5, 10 (2008).
Weller, R. O., Djuanda, E., Yow, H.-Y. & Carare, R. O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 117, 1–14 (2009).
Liu, N.-F., Lu, Q., Jiang, Z.-H., Wang, C.-G. & Zhou, J.-G. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J. Vasc. Surg. 49, 980–987 (2009).
Girard, J.-P., Moussion, C. & Förster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nature Rev. Immunol. 12, 762–773 (2012).
Weller, R. O., Galea, I., Carare, R. O. & Minagar, A. Pathophysiology of the lymphatic drainage of the central nervous system: implications for pathogenesis and therapy of multiple sclerosis. Pathophysiology 17, 295–306 (2010).
Mathieu, E., Gupta, N., Macdonald, R. L., Ai, J. & Yücel, Y. H. In vivo imaging of lymphatic drainage of cerebrospinal fluid in mouse. Fluids Barriers CNS 10, 35 (2013).
Cserr, H. F., Harling-Berg, C. J. & Knopf, P. M. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 2, 269–276 (1992).
Harris, M. G. et al. Immune privilege of the CNS is not the consequence of limited antigen sampling. Sci. Rep. 4, 4422 (2014).
Laman, J. D. & Weller, R. O. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J. Neuroimmune Pharmacol. 8, 840–856 (2013).
Schneider, M., Ny, A., Ruiz de Almodovar, C. & Carmeliet, P. A new mouse model to study acquired lymphedema. PLoS Med. 3, e264 (2006).
Kida, S., Pantazis, A. & Weller, R. O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 19, 480–488 (1993).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Yang, L. et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med. 11, 107 (2013).
Berton, M. et al. Generalized lymphedema associated with neurologic signs (GLANS) syndrome: A new entity? J. Am. Acad. Dermatol. 72, 333–339 (2015).
Akiyama, H. et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421 (2000).
Hohlfeld, R. & Wekerle, H. Immunological update on multiple sclerosis. Curr. Opin. Neurol. 14, 299–304 (2001).
Acknowledgements
We thank S. Smith for editing the manuscript; V. Engelhard for initial discussions and suggestions on lymphatic endothelial cell specific markers. We also thank the members of the Kipnis laboratory, Brain Immunology and Glia (BIG) center, and the Department of Neuroscience at the University of Virginia (especially J. Lukens) for their valuable comments during multiple discussions of this work. L. Holland and B. Lopes provided the human samples. This work was funded by “Fondation pour la Recherche Medicale” to A.L. and by The National Institutes of Health (R01AG034113 and R01NS061973) to J.K.
Author information
Authors and Affiliations
Contributions
A.L. performed most of the experiments, analysed the data, and contributed to experimental design and manuscript writing. I.S. performed all the surgeries and intracerebroventricular injections. T.J.K. assisted with the experiments and the analysis of the data. J.D.E, S.J.R. and J.D.P. participated in the discussions and helped with the experimental design. N.C.D. performed the xDCLN experiment. D.C. contributed to the imaging and the analysis of the electron microscopy images. J.W.M. contributed with data analysis of the human samples. K.S.L. contributed to experimental design and to manuscript editing. T.H.H. assisted to the intravital imaging experiment, contributed to experimental design and to manuscript editing. J.K. designed the study, assisted with data analysis, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
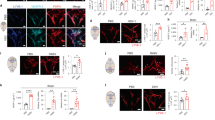
Extended Data Figure 1 Meningeal immunity and lymphatic vessels in the dural sinuses.
a, Representative image of CD31 staining in whole-mount meninges (scale bar, 2,000 µm). b, Representative images of T cells (CD3e, arrowheads) in the dura/arachnoid, pia, dural sinuses, and choroid plexus (scale bars, 70 µm). c, Quantification of T-cell density in different meningeal compartments (mean ± s.e.m.; n = 6 animals each group from 2 independent experiments; ***P <0.001; Kruskal–Wallis test with Dunn’s post hoc test). d, Quantification of MHCII-expressing cells in different meningeal compartments (mean ± s.e.m.; n = 6 animals each group from 2 independent experiments; ***P <0.001; Kruskal–Wallis test with Dunn’s post hoc test). e, Adult mice were injected i.v. with 100 µl of DyLight 488 lectin 5 min before euthanasia to enable labelling of the cardiovascular system. Meninges were harvested and stained with anti-CD3e. Representative orthogonal images of T-cell localization in the lumen (white arrowhead) and outside of the sinus (yellow arrowhead; n = 2 mice; scale bar, 70 µm). f, Adult mice were injected i.v. with 10 µg of FITC-conjugated anti-CD45 antibody or FITC-conjugated isotype antibody. Meninges were harvested one hour after the injection and labelled with anti-CD3e. Representative images of CD3e immunolabelling around dural sinuses are shown. CD45-positive cells do not co-localize with CD3e+ cells (a), suggesting an abluminal localization of the latter (n = 2 mice each group; scale bars, 20 µm). g, Representative 3D reconstruction of the lymphatic vessels localization around the superior sagittal sinus. Adult mice were injected i.v. with 100 µl of DyLight 488 lectin 5 min before euthanasia in order to stain the cardiovascular system. Meninges were harvested and labelled with anti-Lyve-1. The lack of lectin staining in the Lyve-1-positive meningeal lymphatic vessels suggests that they are independent of the cardiovascular system (n = 3 mice; scale bars, left, 50 µm and right, 120 µm). The mounting of the whole meninges results in the flattening of the sinus, thus it does not appear tubular.
Extended Data Figure 2 Identification, characterization and validation of the expression of classical lymphatic endothelial cell markers by the meningeal lymphatic vessels.
a, Representative images of Prox1 labelling on meningeal Lyve-1 expressing vessels (n = 3 mice; scale bars, 10 µm). b, Schematic representation of the whole-mount dissection of the diaphragm. c, Characterization of the specificity of the podoplanin antibody. Representative images of whole-mount diaphragm labelled with anti-Lyve-1 and anti-podoplanin (ci), control isotype (cii) or the anti-podoplanin pre-incubated overnight with a saturated concentration of recombinant podoplanin protein (ciii; scale bars, 20 µm). d, Characterization of the specificity of the VEGFR3 antibody. Representative images of whole-mount diaphragm and dura mater labelled with anti-Lyve-1 and anti-VEGFR3 (di), secondary antibody only (dii), or the anti-VEGFR3 pre-incubated overnight with a saturated concentration of recombinant VEGFR3 protein (diii; scale bars, 20 µm). e, Quantification of the number of Prox1+ nuclei per mm2 of lymphatic vessel (mean ± s.e.m.; n = 4 animals each group).
Extended Data Figure 3 Identification of the meningeal lymphatic endothelial cell population by flow cytometry.
FACS analysis of the lymphatic endothelial cells in diaphragm, skin (ear), and dural sinuses. a, Gating strategy employed to identify lymphatic endothelial cells (CD31+podoplanin+). Lymphatic endothelial cells are identified as singlet, live cells, CD45− and CD31+podoplanin+. b, Representative dot plots for lymphatic endothelial cells (CD31+podoplanin+) in the diaphragm, skin, and dura mater of adult mice.
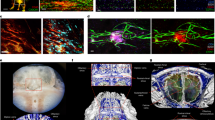
Extended Data Figure 4 Pilot identification of lymphatic vessels in human dura.
a, Representative image of a formalin-fixed coronal section of human superior sagittal sinus. b, c, Representative images of Lyve-1 staining on coronal section of human superior sagittal sinus (scale bar, 100 µm). The box in c highlights the presence of Lyve-1-expressing macrophages in human meninges, as seen in mice. d, Representative images of Lyve-1 and CD68 staining of coronal sections of human superior sagittal sinus. Note the absence of CD68 positivity on Lyve-1 positive structures (scale bars, 50 µm). e, Representative images of podoplanin and Lyve-1 staining of coronal sections of human superior sagittal sinus (scale bars, 50 µm).
Extended Data Figure 5 Initial lymphatic features of meningeal lymphatic vessels.
a, Representative images of CCL21 and Lyve-1 labelling of the meningeal lymphatic vessels (scale bars, 10 µm). b, c, Representative images of VE-Cadherin and Lyve-1 staining on meningeal blood vessels (b) and meningeal lymphatic vessels (c), arrowheads point to the VE-Cadherin aggregates; scale bars, 10 µm). d–f, Representative images of Claudin-5 and Lyve-1 staining on meningeal blood (d) and lymphatic (e) vessels, and diaphragm lymphatic vessels (f); arrowheads point to Claudin-5 aggregates (scale bars, 10 µm). g, h, Representative images of integrin-α9 and Lyve-1 labelling on skin (g; ear) and meninges whole mount (h). Scale bars, 40 µm. No integrin-α9 expressing valves were detected in the meningeal lymphatic vessels. i, Representative low power micrographs (transmission electron microscopy) of the meningeal lymphatic vessels (scale bar, 2 µm); L, lumen; SC, supporting cell; LEC, lymphatic endothelial cell; BEC, sinusal endothelial cell. Red arrowheads point to anchoring filaments. j, Table summarizing morphological features of the lymphatic network in different regions of the meninges and the diaphragm. Diameters are expressed in µm and branching as number of branches per mm of vessel; (mean ± s.e.m.; n = 4 animals each group from 2 independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001; two-way ANOVA with Bonferroni post hoc test). For statistics, the presented comparisons were between the diaphragm and the superior sagittal sinus and between the superior sagittal sinus and the transverse sinuses.
Extended Data Figure 6 Drainage of cerebrospinal fluid into the meningeal lymphatic vessels.
a, Representative z-stack of QDot655 filled cerebrospinal fluid drainage both in the blood vasculature (sinus) and in the meningeal lymphatic vessels after i.c.v. injection (scale bar, 20 µm). b, Representative images of CD31 and Lyve-1 immunostaining on whole-mount meninges. Adult mice were injected i.c.v. with 2.5 µg of Alexa488 conjugated anti-Lyve-1 antibody. Thirty minutes after the injection, the meninges were harvested and stained with anti-CD31. Injected in vivo, the Lyve-1 antibody illuminates the lymphatic vessels (scale bar, 20 µm). c, Representative z-stack of the superior sagittal sinus of adult mice injected i.v. with QDot655 and i.c.v. with Alexa488 conjugated anti-Lyve-1 antibody. ci, Coronal section of the z-stack presented in panel c. The signal from the remaining skull and/or collagen-rich structure above the meninges was recorded (blue). cii, 3D reconstruction of the z-stack presented in panel c showing the localization of the meningeal lymphatic vessels under the skull (scale bars, 50µm).
Extended Data Figure 7 Meningeal lymphatic vessels carrying immune cells.
a, Representative images of T cells (CD3e) and lymphatic endothelial cells (Lyve-1) on dural sinuses (scale bar, 20µm). aii–aiii, Orthogonal sections representing T-cell localization around aii and within aiii the Lyve-1 structures (scale bars, 5µm). b, Quantification of the sinusal T cells and MHCII-expressing cells within the lymphatic vessels (mean ± s.e.m., n = 7–8 mice from 3 independent experiments). c, d, Representative images of Lyve-1 staining on dural meninges from CD11cYFP mice (scale bars, 20 µm). CD11c-positive cells (most probably dendritic cells) can be found inside the meningeal lymphatic vessels. e, Representative image of B220+ cells and lymphatic endothelial cells (Lyve-1) immunolabelling in the meninges (yellow arrowheads indicate B220+CD11c– cells; scale bar, 20 µm). f, Representative dot plots of B220+ cells (gated on singlets, live, CD45+) within the dural sinuses expressing CD19; ∼40% of the B220+ cells express CD19.
Extended Data Figure 8 Drainage of Evans blue from the meningeal lymphatic vessels but not the nasal mucosa into the deep cervical lymph nodes.
a–c, Adult mice were injected i.c.v. with 5 µl of 10% Evans blue. The meninges were harvested 30 min after injection and Evans blue localization was assessed by confocal microscopy. a, Representative images of Evans blue localization in both the sinus and the meningeal lymphatic vessels (n = 9 mice; scale bars, 40 µm). b, Representative profile of Evans blue and Lyve-1 relative fluorescence intensity on a cross-section of the image presented in a. c, Quantification of the average intensity of Evans blue in the sinus, the lymphatic vessels and the meninges of adult mice (mean ± s.e.m., n = 16 analysed fields from 4 independent animals; **P < 0.01, Kruskall–Wallis with Dunn’s multiple comparisons test). d, e, Adult mice were injected intranasally with 5 µl of 10% Evans blue. The successful targeting of the nasal mucosa (d) and the lack of accumulation of Evans blue in the deep cervical lymph nodes (e) 30 min after the injection are demonstrated.
Extended Data Figure 9 Effects of deep cervical lymph node resection and of the lymphatic vessels ligation on the meningeal immune compartment.
a–e, The deep cervical lymph nodes were resected (xDCLN) or sham-operated. Three weeks after resection, the meninges were harvested, single cells isolated, and analysed for T-cell content by flow cytometry. a, Gating strategy to analyse meningeal T cells. Meningeal T cells are selected for singlets, CD45+, live cells and TCRβ+. b, Representative dot plot for CD8+ and CD4+ T cells in meninges of sham and xDCLN mice. c, Quantification of total T cells (TCRβ+), CD4+ and CD8+ in the meninges of xDCLN and sham mice (mean ± s.e.m.; n = 3 animals each group; *P = 0.018; **P = 0.006 (CD8), P = 0.003 (TCRb); Student’s t-test; a representative experiment, out of two independently performed, is presented). d, Representative expression of CD62L and CD44 by CD4+ T cells phenotype in sham and xDCLN mice (n = 3 mice per group). e, Representative histogram for CD71 expression by meningeal CD4+ T cells in sham and xDCLN mice (n = 3 mice per group). f, Representative images of the ligation surgery. To highlight the lymph vessels, Evans blue was injected i.c.v. before the surgery. Black arrowhead points to the node, yellow arrowhead points to the ligated Evans blue-filled vessels. g, Sham-operated or ligated animals were injected i.c.v. with 5 µl of 10% Evans blue. The deep cervical lymph nodes were harvested 30 min after the injection and analysed for Evans blue content. Representative images of the Evans blue accumulation in the deep cervical lymph nodes of the sham-operated and ligated animals are presented. h, Quantification of the meningeal lymphatic vessel diameter in the superior sagittal sinus and the transverse sinuses in sham mice and after ligation of the collecting lymphatic vessels (mean ± s.e.m., n = 5 mice per group from 2 independent experiments; two-way ANOVA with Bonferroni post hoc test).
Extended Data Figure 10 Connection between the glymphatic system and the meningeal lymphatic system.
A schematic representation of a connection between the glymphatic system, responsible for collecting of the interstitial fluids from within the central nervous system parenchyma to cerebrospinal fluid, and our newly identified meningeal lymphatic vessels.
Supplementary information
3D reconstruction of the meningeal lymphatic vessels around the superior sagittal sinus
Adult mice were injected i.v. with DyLight 488 lectin prior to euthanasia. Meninges were harvested and stained for lymphatic endothelial cells (Lyve-1). A 3D reconstruction of both the blood vasculature (green) and the meningeal lymphatic vessels (red) using IMARIS software are presented. (MOV 6838 kb)
Morphological features of the meningeal lymphatic vessels
Meninges and diaphragm from adult mice were harvested and stained for lymphatic endothelial cells (Lyve-1). A 3D reconstruction of the lymphatic network using IMARIS software is presented. (MP4 5998 kb)
CSF-filled vessel lining the dural sinuses
Adult mice were injected i.v. with fluorescein and i.c.v. with QDot655. The superior sagittal sinus was imaged intravitally by 2-photon microscope under a thinned skull. The CSF-filled vessel (red) is visible between the sinus (left) and the CSF (right). (MOV 3465 kb)
3D reconstruction of the meningeal lymphatic vessel by multiphoton microscopy
Adult mice were injected i.v. with QDot655 (red) and i.c.v. with Alexa488 conjugated anti-Lyve-1 antibody (green). The superior sagittal sinus was imaged intravitally by 2-photon microscope under a thinned skull and the surface was reconstructed using IMARIS software. The signal from the remaining skull (blue) was recorded. (MOV 8953 kb)
Flow directionality of the meningeal lymphatics
Adult mice were injected i.v. with fluorescein and i.c.v. with QDot655. The superior sagittal sinus was imaged intravitally by 2-photon microscope under a thinned skull. The blood flow in the sinus (green, left) appears faster than the one in the meningeal lymphatic vessels (red, right). The flow inside of the lymphatic vessels appears unidirectional. (MOV 9966 kb)
Rights and permissions
About this article
Cite this article
Louveau, A., Smirnov, I., Keyes, T. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). https://doi.org/10.1038/nature14432
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14432
This article is cited by
-
Neuroimmunology and ageing – the state of the art
Immunity & Ageing (2024)
-
Blood pressure lowering enhances cerebrospinal fluid efflux to the systemic circulation primarily via the lymphatic vasculature
Fluids and Barriers of the CNS (2024)
-
Large-scale in-silico analysis of CSF dynamics within the subarachnoid space of the optic nerve
Fluids and Barriers of the CNS (2024)
-
Enhanced meningeal lymphatic drainage ameliorates lipopolysaccharide-induced brain injury in aged mice
Journal of Neuroinflammation (2024)
-
Postnatal meningeal CSF transport is primarily mediated by the arachnoid and pia maters and is not altered after intraventricular hemorrhage-posthemorrhagic hydrocephalus
Fluids and Barriers of the CNS (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.