Abstract
How cells acquire their fate is a fundamental question in developmental and regenerative biology. Multipotent progenitors undergo cell-fate restriction in response to cues from the microenvironment, the nature of which is poorly understood. In the case of the lymphatic system, venous cells from the cardinal vein are thought to generate lymphatic vessels through trans-differentiation. Here we show that in zebrafish, lymphatic progenitors arise from a previously uncharacterized niche of specialized angioblasts within the cardinal vein, which also generates arterial and venous fates. We further identify Wnt5b as a novel lymphatic inductive signal and show that it also promotes the ‘angioblast-to-lymphatic’ transition in human embryonic stem cells, suggesting that this process is evolutionarily conserved. Our results uncover a novel mechanism of lymphatic specification, and provide the first characterization of the lymphatic inductive niche. More broadly, our findings highlight the cardinal vein as a heterogeneous structure, analogous to the haematopoietic niche in the aortic floor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Alitalo, K. The lymphatic vasculature in disease. Nature Med. 17, 1371–1380 (2011).
Sabin, F. R. On the origin of the lymphatic system from the veins, and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1, 367–389 (1902).
Huntington, G. & McClure, C. The anatomy and development of the jugular lymph sac in the domestic cat (Felis domestica). Am. J. Anat. 10, 177–312 (1910).
Yaniv, K. et al. Live imaging of lymphatic development in the zebrafish. Nature Med. 12, 711–716 (2006).
Srinivasan, R. S. et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 21, 2422–2432 (2007).
Ny, A. et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nature Med. 11, 998–1004 (2005).
Wilting, J., Tomarev, S. I., Christ, B. & Schweigerer, L. Lymphangioblasts in embryonic lymphangiogenesis. Lymphat. Res. Biol. 1, 33–40 (2003).
Yang, Y. & Oliver, G. Development of the mammalian lymphatic vasculature. J. Clin. Invest. 124, 888–897 (2014).
Wigle, J. T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002).
Francois, M. et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 456, 643–647 (2008).
Srinivasan, R. S. et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 24, 696–707 (2010).
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature Immunol. 5, 74–80 (2004).
Dunworth, W. P. et al. Bone morphogenetic protein 2 signaling negatively modulates lymphatic development in vertebrate embryos. Circ. Res. 114, 56–66 (2014).
Deng, Y., Atri, D., Eichmann, A. & Simons, M. Endothelial ERK signaling controls lymphatic fate specification. J. Clin. Invest. 123, 1202–1215 (2013).
Küchler, A. M. et al. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr. Biol. 16, 1244–1248 (2006).
Avraham-Davidi, I. et al. ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nature Med. 18, 967–973 (2012).
Herwig, L. et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr. Biol. 21, 1942–1948 (2011).
Lim, A. H. et al. Motoneurons are essential for vascular pathfinding. Development 138, 3847–3857 (2011).
Isogai, S., Lawson, N. D., Torrealday, S., Horiguchi, M. & Weinstein, B. M. Angiogenic network formation in the developing vertebrate trunk. Development 130, 5281–5290 (2003).
Kohli, V., Schumacher, J. A., Desai, S. P., Rehn, K. & Sumanas, S. Arterial and venous progenitors of the major axial vessels originate at distinct locations. Dev. Cell 25, 196–206 (2013).
Herbert, S. P. et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 326, 294–298 (2009).
Conway, E. M., Collen, D. & Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 49, 507–521 (2001).
Vatine, G. D. et al. Zebrafish as a model for monocarboxyl transporter 8-deficiency. J. Biol. Chem. 288, 169–180 (2013).
Okuda, K. S. et al. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139, 2381–2391 (2012).
Hashimshony, T., Wagner, F., Sher, N. & Yanai, I. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2, 666–673 (2012).
van Impel, A. et al. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 141, 1228–1238 (2014).
Okamura, M. et al. COUP-TFII acts downstream of Wnt/β-catenin signal to silence PPARγ gene expression and repress adipogenesis. Proc. Natl Acad. Sci. USA 106, 5819–5824 (2009).
Karalay, O. et al. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc. Natl Acad. Sci. USA 108, 5807–5812 (2011).
Cermenati, S. et al. Sox18 genetically interacts with VegfC to regulate lymphangiogenesis in zebrafish. Arterioscler. Thromb. Vasc. Biol. 33, 1238–1247 (2013).
Hogan, B. M. et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nature Genet. 41, 396–398 (2009).
Yamashita, J. et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408, 92–96 (2000).
Kusuma, S. et al. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl Acad. Sci. USA 110, 12601–12606 (2013).
Mikels, A. J. & Nusse, R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 4, e115 (2006).
van Amerongen, R., Fuerer, C., Mizutani, M. & Nusse, R. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev. Biol. 369, 101–114 (2012).
Ikeda, S. et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 17, 1371–1384 (1998).
Veien, E. S., Grierson, M. J., Saund, R. S. & Dorsky, R. I. Expression pattern of zebrafish tcf7 suggests unexplored domains of Wnt/beta-catenin activity. Dev. Dyn. 233, 233–239 (2005).
Lindskog, H. et al. Molecular identification of venous progenitors in the dorsal aorta reveals an aortic origin for the cardinal vein in mammals. Development 141, 1120–1128 (2014).
Stoick-Cooper, C. L. et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134, 479–489 (2007).
Moro, E. et al. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev. Biol. 366, 327–340 (2012).
Alexander, J., Rothenberg, M., Henry, G. L. & Stainier, D. Y. casanova plays an early and essential role in endoderm formation in zebrafish. Dev. Biol. 215, 343–357 (1999).
Lele, Z., Bakkers, J. & Hammerschmidt, M. Morpholino phenocopies of the swirl, snailhouse, somitabun, minifin, silberblick, and pipetail mutations. Genesis 30, 190–194 (2001).
Heisenberg, C. P. et al. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 15, 1427–1434 (2001).
Hurlstone, A. F. et al. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature 425, 633–637 (2003).
Bussmann, J. et al. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 137, 2653–2657 (2010).
Fisher, S. et al. Evaluating the biological relevance of putative enhancers using Tol2 transposon-mediated transgenesis in zebrafish. Nature Protocols 1, 1297–1305 (2006).
Villefranc, J. A., Amigo, J. & Lawson, N. D. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 (2007).
Cermenati, S. et al. Sox18 and Sox7 play redundant roles in vascular development. Blood 111, 2657–2666 (2008).
Shepard, J. L., Stern, H. M., Pfaff, K. L. & Amatruda, J. F. Analysis of the cell cycle in zebrafish embryos. Methods Cell Biol. 76, 109–125 (2004).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature Chem. Biol. 5, 100–107 (2009).
Cirone, P. et al. A role for planar cell polarity signaling in angiogenesis. Angiogenesis 11, 347–360 (2008).
Griffin, K. J. & Kimelman, D. One-Eyed Pinhead and Spadetail are essential for heart and somite formation. Nature Cell Biol. 4, 821–825 (2002).
Nyholm, M. K., Wu, S. F., Dorsky, R. I. & Grinblat, Y. The zebrafish zic2a-zic5 gene pair acts downstream of canonical Wnt signaling to control cell proliferation in the developing tectum. Development 134, 735–746 (2007).
Dorsky, R. I., Sheldahl, L. C. & Moon, R. T. A transgenic Lef1/β-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev. Biol. 241, 229–237 (2002).
Shimizu, T. et al. Cooperative roles of Bozozok/Dharma and Nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech. Dev. 91, 293–303 (2000).
Kim, H. J. et al. Wnt5 signaling in vertebrate pancreas development. BMC Biol. 3, 23 (2005).
Ben Shoham, A. et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development 139, 3859–3869 (2012).
Levin, M., Hashimshony, T., Wagner, F. & Yanai, I. Developmental milestones punctuate gene expression in the Caenorhabditis embryo. Dev. Cell 22, 1101–1108 (2012).
Bauer, S., Grossmann, S., Vingron, M. & Robinson, P. N. Ontologizer 2.0–a multifunctional tool for GO term enrichment analysis and data exploration. Bioinformatics 24, 1650–1651 (2008).
Levin, M. A novel immunohistochemical method for evaluation of antibody specificity and detection of labile targets in biological tissue. J. Biochem. Biophys. Methods 58, 85–96 (2004).
Jin, S. W., Beis, D., Mitchell, T., Chen, J. N. & Stainier, D. Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209 (2005).
Acknowledgements
The authors would like to thank B. Cohen, N. Strasser, R. Solomon and F. Bochner for technical assistance, N. Stettner and A. Harmelin for animal care, G. Beck and E. Ainbinder for assistance with hESC experiments, E. Winter for RNA-Seq analyses, F. Argenton for providing the Tg(7xTCF-Xla.Siam:nlsmCherry)ia5 transgenic line, G. Weidinger for the Tg(hsp70l:wnt5b-GFP)w33 line, E. Ober for the TgBAC(prox1a:KalT4-UAS:uncTagRFP)nim5 line, S. Schulte-Merker for the Tg(flt4BAC:mCitrine)hu7135 line, S. Sumanas for the Tg(etv2:GFP)ci1 line, M. Affolter and H. G. Belting for the Tg(fli1:gal4ubs3;uasKaederk8) line, A. Inbal for the pCS2-axin plasmid, B. Weinstein for the pME-nr2f2 plasmid and the cas mutants, M. Beltrame for the pCMV sox18 plasmid, and E. Tzahor, E. Zelzer, M. Neeman and B. Shilo for critical reading of the manuscript. The authors are grateful to all the members of the Yaniv laboratory for discussion, technical assistance and continuous support. This work was supported in part by Marie Curie Actions-International Reintegration grants FP7-PEOPLE-2009-RG 256393 (to K.Y.), Minerva Foundation 711128 (to K.Y.), German-Israeli Foundation Young Investigator Program 1967/2009 (to K.Y.), Israel Cancer Research Foundation Postdoctoral Fellowship (to G.M.), Lymphatic Research and Education Network postdoctoral fellowship (to G.M.), Northrine Westphalia Return fellowship (to W.H.), US National Institutes of Health (NIH) R01 HL122599 (to N.D.L.), JSPS Postdoctoral Fellowships for Research Abroad (to M.S.), ERC 310927 (to I.Y.). K.Y. is supported by the Karen Siem Fellowship for Women in Science; the Willner Family Center for Vascular Biology; the estate of Paul Ourieff; the Carolito Stiftung; Lois Rosen, Los Angeles, CA; and the Adelis Foundation. K.Y. is the incumbent of the Louis and Ida Rich Career Development Chair.
Author information
Authors and Affiliations
Contributions
J.N. and G.M. designed and conducted experiments, analysed data, and co-wrote the manuscript; Y.S. designed and conducted experiments on human ESCs and analysed data; T.L, L.A., O.M., A.J.-V. and M.S. conducted experiments and data analyses; I.A.-D. and V.K. conducted in vitro experiments, N.S. and T.H. conducted RNA-Seq experiments and data analyses; R.H. assisted with animal care and genotyping; L.G.-B. and J.W.A. generated transgenic lines; G.A. managed the fish facility; S.B-D. performed bioinformatics analyses; O.G. assisted with image processing analyses; P.S.C. provided the Tg(lyve1:EGFP)nz150 and Tg(lyve1:dsRed2)nz101 transgenic lines. W.H. and N.D.L. designed and supervised part of the experiments; I.Y. designed and supervised RNA-Seq experiments; J.H.H. supervised part of the hESCs experiments; K.Y. initiated and directed the study, designed experiments, analysed data and co-wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
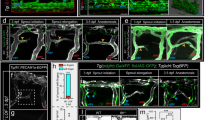
Extended Data Figure 1 Mesoderm-derived angioblasts generate LECs through asymmetric cell division.
a, Snapshots from a time-lapse sequence of a Tg (fli1:nEGFP)y7 zebrafish embryo, showing the origin of a PAC cell (yellow) in the vPCV (nimaged embryos = 7). b, vPCV (left panel), and dPCV (right panel) Kaede photoconverted cells at 48 hpf. c, Kaede-photoswitched ‘medial’ (left panel) and ‘early lateral’ (right panel) angioblasts. d, Snapshots from a time-lapse sequence of a plcg1 mutant, showing the origin of a PAC cell (green) in the vPCV (nimaged embryos = 3). e, Quantification of symmetric and asymmetric division events in the vPCV and dPCV of double Tg(flt1_9a_cFos:GFP; lyve1:dsRed2 nz101) embryos (nimaged embryos = 6). Scale bars, 30 μm.
Extended Data Figure 2 Analysis of cell division in the zebrafish axial vessels.
a, Phospho-histone H3 staining shows no difference in the number of proliferative endothelial cells among the DA, dPCV and vPCV (n24 hpf embryos = 17, n26 hpf embryos = 16, n28 hpf embryos = 16, n30 hpf embryos = 16). b, Ectopic induction of Wnt5b in Tg(hsp70l:wnt5b; fli1:EGFP) does not result in enhanced proliferation of endothelial cells (26 hpf; ncontrol embryos = 15, nhsp70:wnt5b embryos = 8, 28 hpf; ncontrol embryos = 14, nhsp70:wnt5b embryos = 8, 30 hpf; ncontrol embryos = 14, nhsp70:wnt5b embryos = 10). Scale bar, 60 μm. Error bars, mean ± s.e.m.
Extended Data Figure 3 Fate map analysis of vPCV cells.
a, Schematic representation of the subintestinal plexus at 72 hpf. Subintestinal vein (SIV, green), interconnecting SI vessels (purple), supraintestinal artery (SIA, pink), posterior cardinal vein (PCV, blue), dorsal aorta (DA, red). b, Quantification of the number of intersegmental arteries (ISA) and intersegmental veins (ISV) in the first four segments of Tg(flt1_9a_cFos:GFP; lyve1:dsRed2) double transgenic embryos (nembryos = 41). IS# denotes the position of intersegmental vessel. c, Confocal images of Tg(lyve1:dsRed2) (left panel) and Tg(flt1_9a_cFos:GFP; lyve1:dsRed2) (right panel) embryos showing lyve1:dsred2+ endothelial cells in PACs, venous intersegmental vessels (ISVs), PCV and SIV and flt1_9a:GFP+ endothelial cells in the SIA. d, flt1_9a:GFP+ vPCV angioblast (light-blue arrowhead), divides asymmetrically (curved arrow) to generate cells that populate the SIV (31.5 hpf, white arrowhead), and the SIA (53.5 hpf, white arrowhead). Scale bar, 30 µm. Error bars, mean ± s.e.m.
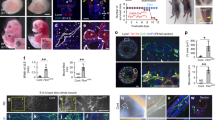
Extended Data Figure 4 Transcriptional profiling of vPCV angioblasts.
a, Experimental setup used for RNA sequencing analysis of FACS isolated vPCV and dPCV cells. b, FACS isolation of green vs red (photoconverted) endothelial cells from Tg(fli1:gal4;uasKaede) embryos following photoswitching of dorsal or ventral PCV (nindependent experiments = 4). c, qRT–PCR analysis of selected candidates shows enrichment in ventral vs dorsal PCV cells (nindependent experiments = 2). d, Gene Ontology enrichment in vPCV vs dPCV cells (results represent 2 out of 4 independent biological repeats). Error bars, geometrical mean ± s.e.g.m.
Extended Data Figure 5 Endoderm-derived Wnt5b is required for lymphatic development.
a, PAC-containing segments in WT (arrows) and cas mutants (asterisks). b, In situ hybridization at 20 hpf showing expression of wnt5b mRNA (blue arrowhead) in the endoderm of WT embryos. c, PAC-containing segments in uninjected (UI) (arrows) and wnt5b MO-injected embryos (asterisks). d, ppt mutants injected with wnt5b MO (subdose) display significant reduction in PAC number (nUI embryos = 38, nwnt5b-MO embryos sub = 38, nppt-UI embryos = 34, nppt, wnt5b MO sub-embryos = 34; *P = 1.2 × 10−5). e, wnt5b morphants exhibit marked reduction in the number of thoracic duct-containing segments (asterisks) as compared to uninjected (UI) siblings (arrows) (nUI-embryos = 38, nwnt5b MO-embryos = 32; *P = 4.5 × 10−30). f, The number of flt1+ vPCV progenitors is not affected in wnt5b morphants (nUI-embryos = 31, nwnt5b-MO embryos = 31). Scale bars, a, c, 60 μm; b, e, f, 30 μm. Error bars, mean ± s.e.m.
Extended Data Figure 6 Wnt5b is not required for sprouting from the PCV.
a, Phenotypic analysis of Wnt5b overexpression in Tg(hsp70l:wnt5b-GFP; lyve1:dsRed2) embryos, following 25–30 min heat shock (HS), at 23, 25 and 27 hpf (23 hpf embryos nHS-25 min = 18, nHS-30 min = 14, nHS-40 min = 15, 25 hpf embryos nHS-25 min = 14; nHS-30 min = 17, nHS-40 min = 20, 27 hpf embryos nHS-25 min = 19, nHS-30 min = 17, nHS-40 min = 10). b, The number of vISVs vs aISVs is unaltered in wnt5b morphants as compared to Control MO-injected siblings (nControl MO-embryos = 43, nwnt5b MO-embryos = 41). c, flt1_9a+ vPCV cells are detected in the supraintestinal artery (SIA) and subintestinal vein (SIV) of wnt5b MO-injected embryos (nCtrl MO = 16, nwnt5b MO = 16). Scale bars, 60 μm. Error bars, mean ± s.e.m.
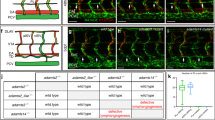
Extended Data Figure 7 Wnt5b induces the “angioblast-to-lymphatic” specification.
a, Selected frames from a time-lapse sequence of a Tg(fli1:gal4;uasKaede) embryo injected with wnt5b MO. Photoconverted vPCV cell (white arrow) divides normally (arrows at 48 hpf point to 2 daughter cells), but does not engage in dorsal migration to generate PACs. b, In situ hybridization of Ctrl MO-, and wnt5b MO-injected zebrafish at 30 hpf, with lyve1, sox18, nr2f2 and cdh5 probes, showing specific decrease in lymphatic marker expression in the floor of the PCV (white arrowheads) of wnt5b morphants. The pan-endothelial marker cdh5, as well as the arterial expression of sox18, remain unchanged in wnt5b morphants. c, vegfc and ccbe1 mRNA levels remain unaltered in sox32 and wnt5b morphants. d, Immunostaining of Prox1 shows marked increase in protein levels following ectopic activation of Wnt5b in Tg(hsp70l:wnt5b; fli1:EGFP) embryos (co-localization channel is shown in yellow, white arrowheads). e, qRT–PCR analysis of FLT4 and CDH5 in hESCs treated with WNT5B (nindependent-experiments = 3; *P = 0.03 by one sample t-test). Scale bars, 60 μm. Error bars, geometrical mean ± s.e.g.m.
Extended Data Figure 8 Wnt5b induces LEC specification through activation of canonical pathway.
a, PAC-containing segments (arrows) in wnt5b MO-injected mbl mutants (nwnt5bMO = 42, nmbl;wnt5bMO = 52; *P = 3.4 × 10−10). b, apc mutants (nWT = 18, napc = 19; *P = 0.0006), c, axin1 mRNA-injected embryos (nUI = 33, naxin-mRNA = 46; *P = 1.73 × 10−14), and d, IWR-1 treated embryos (nDMSO = 55, nIWR = 54; *P = 1.05 × 10−21). Scale bars, 60 μm. Error bars, mean ± s.e.m.
Extended Data Figure 9 Involvement of Tcf transcription factors in LEC specification.
a, PAC number remains unchanged in TNP-470 treated Tg(fli1:EGFP) embryos as compared to DMSO (control) (nDMSO = 19, nTNP-470 = 38). b, c, Quantification of PAC-containing segments in the trunk of UI, tcf7, lef1 and tcf4 MO-injected embryos (nUI-embryos = 59, ntcf7-MO embryos = 33, nlef1-MO embryos = 16, ntcf4-MO embryos = 25; *P = 4.53 × 10−25, **P = 9.62 × 10−8, ***P = 9.12 × 10−9). d, Photoswitching of vPCV cells in tcf7 MO-injected Tg(fli1:gal4;uasKaede) embryos (white arrowheads) at 24 hpf. At 48 hpf photoconverted, red vPCV cells (arrowheads) remain in the PCV and do not generate PACs. Scale bars, 30 μm. Error bars, mean ± s.e.m.
Extended Data Figure 10 Wnt5b-dependent activation of β-catenin/TCF in vPCV angioblasts.
a, Selected frames from a time-lapse sequence showing β-catenin/TCF activity in a single vPCV angioblast (light-blue arrowhead), which generates PACs (white arrowhead) through asymmetric cell division (n = 2). b, Confocal images of the trunks of Tg(7xTCFXla.Siam:nlsmCherry; fli1:EGFP) double transgenic zebrafish injected with wnt5b MO, showing decreased β-catenin/TCF activation in vPCV cells (quantified in c) (nUI-embryos = 18, nwnt5b-embryos = 17; *P = 4 × 10−5). Purple signal depicts co-localization of cytoplasmic EGFP and nuclear mCherry. Scale bars, 30 μm. Error bars, mean ± s.e.m.
Supplementary information
LEC progenitors originate in the floor of the PCV
This video shows time-lapse images of the trunk of a Tg(fli:EGFP) zebrafish between 24hpf-58hpf. Shown are two combined panels: the original images are on the left. On the right, a selected LEC progenitor was colored off-line in green to facilitate its visualization. Note its initial location at the ventral PCV (vPCV). (MP4 12837 kb)
LEC progenitors originate in the floor of the PCV in plcg1 mutant
This video shows time-lapseimages of the trunk of a plcg1 mutant, between 24hpf-50hpf. Shown are two combined panels: the original images are on the left. On the right, a selected LEC progenitor was colored off-line in green to facilitate its visualization. Note its initial location at the vPCV (green). Following asymmetric division, a daughter cell (blue), migrates dorsally to generate a PAC sprout. (MP4 15543 kb)
vPCV cells generate LECs through asymmetric cell division
This video shows time-lapse images of a photoswitched vPCV cell in the trunk of Tg(fli1:gal4;uasKaede) embryo between 25hpf-48hpf. Light-blue arrowhead points to a vPCV angioblast; white arrowhead points to daughter cell that generates PAC. The first frame was acquired before photoswitching. (MP4 6974 kb)
LECs arise from a pool of specialized angioblasts
This video shows time-lapse images of the trunk of Tg(flt1_9a_cFos:GFP; lyve1:dsRed) double ransgenic embryo between 30hpf-48hpf. Light-blue arrowheads point to flt1_9a:GFP+ vPCV angioblast; white arrowheads point to flt1_9a:GFP+ daughter cells that generate PACs, downregulate flt1_9a:GFP expression and upregulate lyve1:dsRed expression. (MP4 3896 kb)
PACs arise from prox1a-expressing LEC progenitors
This video shows time-lapse images of the trunk of Tg(fli1:EGFP; prox1a:KalT4-UAS:uncTagRFP) double transgenic embryo between 23-55 hpf. Cells showing co-localization were pseudo-coloredin yellow. The first cells expressing Prox1a are visible at ~22 hpf in the vPCV. Later on these cells divide and generate progeny that translocates dorsally and buds from the PCV to generate PACs. (MP4 7925 kb)
LEC progenitors do not generate PACs in wnt5b-MO injected embryo
This video shows time-lapse images of the trunk of a g(fli1a:nEGFP; fli1:dsRed) double transgenic embryo injected with wnt5b MO between 28hpf-44hpf. Shown are two combined panels: the original images are on the left.On the right panel, vPCV (colored) cells do not engage in dorsal migration to generate PACs, but undergo normal cell division. (MP4 5355 kb)
Rights and permissions
About this article
Cite this article
Nicenboim, J., Malkinson, G., Lupo, T. et al. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 522, 56–61 (2015). https://doi.org/10.1038/nature14425
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14425
This article is cited by
-
The cytoskeleton adaptor protein Sorbs1 controls the development of lymphatic and venous vessels in zebrafish
BMC Biology (2024)
-
Copper nanoparticles and silver nanoparticles impair lymphangiogenesis in zebrafish
Cell Communication and Signaling (2024)
-
Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Single-cell transcriptomic analysis of vascular endothelial cells in zebrafish embryos
Scientific Reports (2022)
-
Podoplanin is Responsible for the Distinct Blood and Lymphatic Capillaries
Cellular and Molecular Bioengineering (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.