Abstract
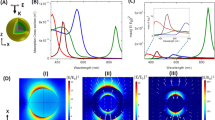
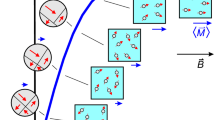
Fluorescent and plasmonic labels and sensors have revolutionized molecular biology, helping visualize cellular and biomolecular processes1,2,3. Increasingly, such probes are now being designed to respond to wavelengths in the near-infrared region, where reduced tissue autofluorescence and photon attenuation enable subsurface in vivo sensing4. But even in the near-infrared region, optical resolution and sensitivity decrease rapidly with increasing depth. Here we present a sensor design that obviates the need for optical addressability by operating in the nuclear magnetic resonance (NMR) radio-frequency spectrum, where signal attenuation and distortion by tissue and biological media are negligible, where background interferences vanish, and where sensors can be spatially located using standard magnetic resonance imaging (MRI) equipment. The radio-frequency-addressable sensor assemblies presented here comprise pairs of magnetic disks spaced by swellable hydrogel material; they reversibly reconfigure in rapid response to chosen stimuli, to give geometry-dependent, dynamic NMR spectral signatures. The sensors can be made from biocompatible materials, are themselves detectable down to low concentrations, and offer potential responsive NMR spectral shifts that are close to a million times greater than those of traditional magnetic resonance spectroscopies. Inherent adaptability should allow such shape-changing systems to measure numerous different environmental and physiological indicators, thus providing broadly generalizable, MRI-compatible, radio-frequency analogues to optically based probes for use in basic chemical, biological, medical and engineering research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhang, J., Campbell, R. E., Ting, A. Y. & Tsien, R. Y. Creating new fluorescent probes for cell biology. Nature Rev. Mol. Cell Biol. 3, 906–918 (2002); erratum. 4, 80 (2003)
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–554 (2005)
Anker, J. N. et al. Biosensing with plasmonic nanosensors. Nature Mater. 7, 442–453 (2008)
Hilderbrand, S. A. & Weissleder, R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 14, 71–79 (2010)
Yoo, B. & Pagel, M. D. An overview of responsive MRI contrast agents for molecular imaging. Front. Biosci. 13, 1733–1752 (2008)
Martinez, G. V. et al. Imaging the extracellular pH of tumors by MRI after injection of a single cocktail of T1 and T2 contrast agents. NMR Biomed. 24, 1380–1391 (2011)
Schröder, L., Lowery, T. J., Hilty, C., Wemmer, D. E. & Pines, A. Molecular imaging using a targeted magnetic resonance hyperpolarized biosensor. Science 314, 446–449 (2006)
Woods, M., Woessner, D. E. & Sherry, A. D. Paramagnetic lanthanide complexes as PARACEST agents for medical imaging. Chem. Soc. Rev. 35, 500–511 (2006)
Ward, K. M. & Balaban, R. S. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST). Magn. Reson. Med. 44, 799–802 (2000)
Zabow, G., Dodd, S., Moreland, J. & Koretsky, A. Micro-engineered local field control for high-sensitivity multispectral MRI. Nature 453, 1058–1063 (2008)
Zabow, G., Dodd, S. J., Moreland, J. & Koretsky, A. P. The fabrication of uniform cylindrical shells and their use as spectrally tunable MRI contrast agents. Nanotechnology 20, 385301 (2009)
Zabow, G., Dodd, S. J. & Koretsky, A. P. Ellipsoidal microcavities: electromagnetic properties, fabrication, and use as multispectral MRI agents. Small 10, 1902–1907 (2014)
Wang, X., Wang, C., Anderson, S. & Zhang, X. Microfabricated iron oxide particles for tunable, multispectral magnetic resonance imaging. Mater. Lett. 110, 122–126 (2013)
Wang, C., Wang, X., Anderson, S. W. & Zhang, X. Biocompatible, micro- and nano-fabricated magnetic cylinders for potential use as contrast agents for magnetic resonance imaging. Sens. Actuators B Chem. 196, 670–675 (2014)
Peppas, N. A., Hilt, J. Z., Khademhosseini, A. & Langer, R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 18, 1345–1360 (2006)
Pan, J. W., Hamm, J. R., Rothman, D. L. & Shulman, R. G. Intracellular pH in human skeletal muscle by 1H NMR. Proc. Natl Acad. Sci. USA 85, 7836–7839 (1988)
Taylor, J. S. & Deutsch, C. Fluorinated α-methylamino acids as 19F NMR indicators of intracellular pH. Biophys. J. 43, 261–267 (1983)
Moon, R. B. & Richards, J. H. Determination of intracellular pH by 31P magnetic resonance. J. Biol. Chem. 248, 7276–7278 (1973)
Wu, Y., Soesbe, T. C., Kiefer, G. E., Zhao, P. & Sherry, A. D. A responsive europium(III) chelate that provides a direct readout of pH by MRI. J. Am. Chem. Soc. 132, 14002–14003 (2010)
Gallagher, F. A. et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 453, 940–943 (2008)
Jindal, A. K. et al. Hyperpolarized 89Y complexes as pH sensitive NMR probes. J. Am. Chem. Soc. 132, 1784–1785 (2010)
Wu, Y. et al. Polymeric PARACEST agents for enhancing MRI contrast sensitivity. J. Am. Chem. Soc. 130, 13854–13855 (2008)
Aime, S., Delli Castelli, D. & Terreno, E. Supramolecular adducts between poly-L-arginine and [TmIIIdotp]: a route to sensitivity-enhanced magnetic resonance imaging-chemical exchange saturation transfer agents. Angew. Chem. Int. Ed. 42, 4527–4529 (2003)
Zabow, G., Koretsky, A. P. & Moreland, J. Design and fabrication of a micromachined multispectral magnetic resonance imaging agent. J. Micromech. Microeng. 19, 025020 (2009)
Sun, J.-Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012)
Byrne, M. E., Park, K. & Peppas, N. A. Molecular imprinting within hydrogels. Adv. Drug Deliv. Rev. 54, 149–161 (2002)
Fischel-Ghodsian, F., Brown, L., Mathiowitz, E., Brandendurg, D. & Langer, R. Enzymatically controlled drug delivery. Proc. Natl Acad. Sci. USA 85, 2403–2406 (1988)
Plunkett, K. N., Berkowski, K. L. & Moore, J. S. Chymotrypsin responsive hydrogel: application of a disulfide exchange protocol for the preparation of methacrylamide containing peptides. Biomacromolecules 6, 632–637 (2005)
Miyata, T., Asami, N. & Uragami, T. A reversibly antigen-responsive hydrogel. Nature 399, 766–769 (1999)
Shapiro, E. M., Skrtic, S. & Koretsky, A. P. Sizing it up: cellular MRI using micron-sized iron oxide particles. Magn. Reson. Med. 53, 329–338 (2005)
Madou, M. J. Fundamentals of Microfabrication: the Science of Miniaturization (CRC Press, 2002)
Kabiri, K., Mirzadeh, H. & Zohuriaan-Mehr, M. J. Undesirable effects of heating on hydrogels. J. Appl. Polym. Sci. 110, 3420–3430 (2008)
Ulijn, R. V. et al. Bioresponsive hydrogels. Mater. Today 10, 40–48 (2007)
Decker, C. & Jenkins, A. D. Kinetic approach of O2 inhibition in ultraviolet- and laser-induced polymerizations. Macromolecules 18, 1241–1244 (1985)
Yoon, J., Cai, S., Suo, Z. & Hayward, R. C. Poroelastic swelling kinetics of thin hydrogel layers: comparison of theory and experiment. Soft Matter 6, 6004–6012 (2010)
Henkelman, R. M., Stanisz, G. J. & Graham, S. J. Magnetization transfer in MRI: a review. NMR Biomed. 14, 57–64 (2001)
Grad, J. & Bryant, R. G. Nuclear magnetic cross-relaxation spectroscopy. J. Magn. Reson. 90, 1–8 (1990)
Jorjani, P. & Ozturk, S. S. Effects of cell density and temperature on oxygen consumption rate for different mammalian cell lines. Biotechnol. Bioeng. 64, 349–356 (1999)
Acknowledgements
This work was supported in part by the NIH NINDS Intramural Research Program. We thank the NIH Mouse Imaging Facility for use of their 14 T MRI, Y. Chen for providing the MDCK cells, and J. Moreland for discussion and NIST Boulder cleanroom access.
Author information
Authors and Affiliations
Contributions
G.Z. conceived of the project, designed the experiments, fabricated the sensors, analysed the data, and wrote the manuscript. S.J.D. designed all NMR/MRI pulse sequences used, helped acquire all NMR/MRI data, and helped write the manuscript. A.P.K. oversaw the work, provided critical feedback, and helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Schematic of microfabrication protocol.
See Methods for explanation of panels a–o.
Extended Data Figure 2 Hydrogel expansion.
Hydrogel sample in compressed (top) and expanded (bottom) state, showing ∼20% linear expansion.
Extended Data Figure 3 Diffusion simulations.
Logarithmically shaded (greyscale bar) plots of numerically simulated ion-concentrations (normalized to initial concentration of unity) due to diffusion between pH buffer solution and water-based agarose gel.
Extended Data Figure 4 Pulse protocol.
Schematic showing off-resonant preparatory pulse train, followed by on-resonant excitation pulse and free-induction-decay (FID) acquisition. The sequence is repeated, each time with different offset frequency, to acquire each point in the z-spectrum.
Extended Data Figure 5 Sensor sensitivity.
z-spectrum, showing magnetization saturated out MS, normalized to the initial water magnetization M0, from sensors with Fe disks of radii 900 nm and thickness 10 nm with resonance around –325 kHz.
Extended Data Figure 6 Sensor miniaturization.
z-spectrum, showing magnetization saturated out MS, normalized to the initial water magnetization M0, for double-disk structures of radii 250 nm with resonance around –525 kHz.
Extended Data Figure 7 Optically probed sensor response rates.
Top panel, a series of consecutive still frames from Supplementary Video 1, showing the propagation of an acid front over an array of sensors. The resulting changes in reflected colours are due to changes in spacing between the top and bottom disks of the sensor, indicating rapid sensor response to introduced acid. Bottom panels, reflected light intensity in green and red channels (normalized to average light intensity across all colour channels) recorded frame-by-frame at the substrate points a and b indicated in leftmost top panel. Slightly different starting and end points within colour oscillation are due to unintentional sensor microfabrication variation across the substrate.
Supplementary information
Video 1: Sensor response to acid pulse (observed through optical intereference)
The video (in real-time at 30 frames per second) shows rapid colour change, which implies rapid sensor response, following the acid front (see online methods). (MP4 1029 kb)
Video S2: Sensor enabled MRI mapping of diffusion-driven spatial ion concentration variations
This animated file, derived from the sensor-recorded ion concentration data was generated by automatically curve-fitting all z-spectra collected, in space and time. The animation comprises a sequential time series of spatial maps of the measured sensor resonant frequencies (see online methods). (MP4 656 kb)
Rights and permissions
About this article
Cite this article
Zabow, G., Dodd, S. & Koretsky, A. Shape-changing magnetic assemblies as high-sensitivity NMR-readable nanoprobes. Nature 520, 73–77 (2015). https://doi.org/10.1038/nature14294
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14294
This article is cited by
-
Quantitative, high-sensitivity measurement of liquid analytes using a smartphone compass
Nature Communications (2024)
-
Non-equilibrium signal integration in hydrogels
Nature Communications (2020)
-
Assessment of the VDW interaction converting DMAPS from the thermal-motion form to the hydrogen-bonded form
Scientific Reports (2019)
-
Biophysical sensing in deep tissue via MRI
Nature Biomedical Engineering (2019)
-
Acoustically modulated magnetic resonance imaging of gas-filled protein nanostructures
Nature Materials (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.