Abstract
Metastatic disease remains the primary cause of death for patients with breast cancer. The different steps of the metastatic cascade rely on reciprocal interactions between cancer cells and their microenvironment. Within this local microenvironment and in distant organs, immune cells and their mediators are known to facilitate metastasis formation1,2. However, the precise contribution of tumour-induced systemic inflammation to metastasis and the mechanisms regulating systemic inflammation are poorly understood. Here we show that tumours maximize their chance of metastasizing by evoking a systemic inflammatory cascade in mouse models of spontaneous breast cancer metastasis. We mechanistically demonstrate that interleukin (IL)-1β elicits IL-17 expression from gamma delta (γδ) T cells, resulting in systemic, granulocyte colony-stimulating factor (G-CSF)-dependent expansion and polarization of neutrophils in mice bearing mammary tumours. Tumour-induced neutrophils acquire the ability to suppress cytotoxic T lymphocytes carrying the CD8 antigen, which limit the establishment of metastases. Neutralization of IL-17 or G-CSF and absence of γδ T cells prevents neutrophil accumulation and downregulates the T-cell-suppressive phenotype of neutrophils. Moreover, the absence of γδ T cells or neutrophils profoundly reduces pulmonary and lymph node metastases without influencing primary tumour progression. Our data indicate that targeting this novel cancer-cell-initiated domino effect within the immune system—the γδ T cell/IL-17/neutrophil axis—represents a new strategy to inhibit metastatic disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Data deposits
RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE55633. Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper. Correspondence and requests for materials should be addressed to K.E.dV. (k.d.visser@nki.nl).
Change history
17 June 2015
The mouse model name in Fig. 1a was corrected.
References
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nature Med. 19, 1423–1437 (2013)
McAllister, S. S. & Weinberg, R. A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nature Cell Biol. 16, 717–727 (2014)
Noh, H., Eomm, M. & Han, A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J. Breast Cancer 16, 55–59 (2013)
Azab, B. et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann. Surg. Oncol. 19, 217–224 (2012)
Granot, Z. et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20, 300–314 (2011)
Kowanetz, M. et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl Acad. Sci. USA 107, 21248–21255 (2010)
Bald, T. et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 507, 109–113 (2014)
Derksen, P. W. et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437–449 (2006)
Doornebal, C. W. et al. A preclinical mouse model of invasive lobular breast cancer metastasis. Cancer Res. 73, 353–363 (2013)
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005)
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009)
Kuonen, F. et al. Inhibition of the Kit ligand/c-Kit axis attenuates metastasis in a mouse model mimicking local breast cancer relapse after radiotherapy. Clin. Cancer Res. 18, 4365–4374 (2012)
Hiratsuka, S. et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2, 289–300 (2002)
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature Cell Biol. 8, 1369–1375 (2006)
Pillay, J., Tak, T., Kamp, V. M. & Koenderman, L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell. Mol. Life Sci. 70, 3813–3827 (2013)
Mazzoni, A. et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695 (2002)
Young, M. R., Wright, M. A., Matthews, J. P., Malik, I. & Prechel, M. Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-β and nitric oxide. J. Immunol. 156, 1916–1922 (1996)
Lejeune, P. et al. Nitric oxide involvement in tumor-induced immunosuppression. J. Immunol. 152, 5077–5083 (1994)
Hamilton, J. A. & Achuthan, A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 34, 81–89 (2013)
Chung, Y. et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30, 576–587 (2009)
Cai, Y. et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35, 596–610 (2011)
Mei, J. et al. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J. Clin. Invest. 122, 974–986 (2012)
Sutton, C. E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009)
Schwarzenberger, P. et al. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J. Immunol. 164, 4783–4789 (2000)
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009)
Han, Y. et al. Prognostic value of chemotherapy-induced neutropenia in early-stage breast cancer. Breast Cancer Res. Treat. 131, 483–490 (2012)
Ma, C. et al. Tumor-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. J. Immunol. 189, 5029–5036 (2012)
Novitskiy, S. V. et al. TGF-β receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov 1, 430–441 (2011)
Chen, W. C. et al. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 63, 225–233 (2013)
Sutherland, T. E. et al. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nature Immunol. 15, 1116–1125 (2014)
de Visser, K. E., Korets, L. V. & Coussens, L. M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7, 411–423 (2005)
Ciampricotti, M., Hau, C. S., Doornebal, C. W., Jonkers, J. & de Visser, K. E. Chemotherapy response of spontaneous mammary tumors is independent of the adaptive immune system. Nature Med. 18, 344–346 (2012)
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001)
Ciampricotti, M. et al. Development of metastatic HER2+ breast cancer is independent of the adaptive immune system. J. Pathol. 224, 56–66 (2011)
Acknowledgements
This work was supported by a Marie Curie Intra-European Fellowship to S.B.C. (BMDCMET 275610); a European Research Council Consolidator award (INFLAMET 615300) to K.E.dV; grants from the Dutch Cancer Society to K.E.dV and J.J. (2011-5004); Worldwide Cancer Research (AICR 11-0677) to K.E.dV; the Netherlands Organization for Scientific Research NWO VIDI (917.96.307) to K.E.dV; and a Dutch Cancer Society/Bas Mulder Award to L.J.A.C.H. (UL2011-5051). We thank J. Borst, T. Schumacher and J. Coquet for discussions. We thank the core facilities at the Netherlands Cancer Institute. We thank L. Coussens for Rag1−/− mice and A. Hayday for Tcrd−/− mice. We thank C. Ries and K. Wartha for technical assistance.
Author information
Authors and Affiliations
Contributions
S.B.C., J.J. and K.E.dV. conceived the ideas and designed the experiments. S.B.C., C.W.D., K.K., J.W., C.H., K.V., N.J.V., M.C., L.J.A.C.H. and K.E.dV. performed the experiments. S.B.C., C.W.D., K.K., J.W., C.H., K.V., N.J.V., L.J.A.C.H. and K.E.dV. analysed the data. S.B.C., K.K. and K.E.dV. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
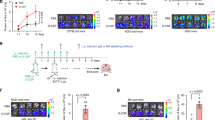
Extended Data Figure 1 Systemic neutrophil expansion and accumulation in mammary tumour-bearing KEP mice and the metastasis model.
a, Representative images of neutrophils identified by the 7/4 antibody in lung sections in WT or KEP mice. Scale bar, 50 μm. b, Quantification of neutrophil accumulation per field of view (FOV) in various organs by immunohistochemistry using the 7/4 antibody (n = 6 WT, 9 KEP mice). c, Absolute neutrophil counts in blood of WT and tumour-bearing KEP mice (n = 4 WT, 8 KEP). d, Quantification of neutrophil accumulation in various organs determined by flow cytometry and gated on CD45+ cells. Neutrophils were not detectable in WT mammary glands (n = 5 WT, 7 KEP mice). e, Representative images of Ly6G-stained lung sections and quantification of neutrophil accumulation in the metastasis model. Data were generated from mock-transplanted, non-tumour-bearing mice (0 mm2), or tumour-transplanted recipient mice killed when tumours reached the tumour size shown or when mice exhibited signs of respiratory distress due to pulmonary metastasis. For quantification in lungs with metastases, neutrophils residing inside metastases were excluded. T, pulmonary metastatic lesion. Scale bar, 100 μm (n = 3, 5, 6, 6 and 3 mice for 0, 9, 25, 100 mm2 and metastasis respectively). f, Kinetics of neutrophil accumulation in various organs of the metastasis model by flow cytometry after gating on CD45+ cells. Recipient mice transplanted with KEP tumour pieces were killed at the tumour size shown (n = 6, 5, 6, and 7 mice for 0, 9, 25, 100 mm2 respectively). g, Kinetics of neutrophil proportions in blood (gated on CD45+ cells), before and after surgical removal of their primary tumour (n = 5). All data are mean + s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 as determined by Mann–Whitney U-test or Kruskal–Wallis test followed by post-hoc Dunn’s test.
Extended Data Figure 2 Neutrophil depletion does not affect primary tumour or metastatic nodule growth.
a, Schematic illustration of the neutrophil depletion experiment in the spontaneous metastasis model. b, Representative dot plots of neutrophils gated on CD45+ cells in blood of control and anti-Ly6G-treated recipient mice. The Gr1 antibody was used here to avoid false-negative results since the anti-Ly6G depleting antibody may mask the Ly6G epitope. CD11b+Gr1high cells were Ly6C+CCR2−, indicating that these cells were neutrophils. CD11b+Gr1low cells that were Ly6C+ and CCR2+ represented the monocytic fraction. c, Quantification of neutrophil depletion in blood of control and anti-Ly6G-treated recipient mice at the tumour size indicated (n = 8 control, 5 anti-Ly6G; **P < 0.01 as determined by Mann–Whitney U-test). d, Primary tumour growth kinetics of mice treated as indicated (n = 12 control, 14 anti-Ly6G). e, Representative images of primary tumours in the metastasis model treated as shown and stained with haematoxylin and eosin, cytokeratin 8, vimentin, E-cadherin and CD34. Scale bar, 100 μm. f, Quantification of blood vessels per field of view (FOV) in control and neutrophil-depleted mice by anti-CD34 immunohistochemistry (n = 10). g, Quantification of pulmonary metastatic nodule size in control and neutrophil-depleted mice (n = 9 control, 11 early phase, 14 late phase mice). All data are mean + s.e.m.
Extended Data Figure 3 Subpopulations of neutrophils in mammary tumour-bearing mice are immature.
a, Gating strategy for identification of neutrophils (CD45+CD11b+Ly6G+Ly6C+F4/80− cells), cKIT+ neutrophils and monocytes (CD45+CD11b+Ly6G−Ly6C+F4/80− cells) by flow cytometry. Blood cells from WT and tumour-bearing KEP mice are shown here. b, Quantification of cKIT+ neutrophil accumulation in various organs determined by flow cytometry after gating on CD45+CD11b+Ly6G+Ly6C+F4/80− cells. cKIT+ neutrophils were not detectable in WT mammary glands (n = 5 WT, 7 KEP; Mann–Whitney U-test). c, cKIT+ neutrophil proportions in various organs of the metastasis model as determined by flow cytometry after gating on CD45+CD11b+Ly6G+Ly6C+F4/80− cells. Mice were killed at the tumour size shown (n = 5, 5, 5, and 8 mice for 0, 9, 25, 100 mm2 respectively; Kruskal–Wallis test followed by post-hoc Dunn’s test). d, Kinetics of cKIT+ neutrophil proportions in blood (gated on CD45+CD11b+Ly6G+Ly6C+F4/80− cells), before and after surgical removal of the primary tumour (n = 5 per group; Mann–Whitney U-test). e, Representative images and quantification of neutrophil nuclear morphology. Ly6G+ cells were isolated from blood of WT and tumour-bearing KEP mice then assessed by Giemsa stain. Hyper-segmented cells were considered mature, whereas all other cells were considered immature. Scale bar, 10 μm. (n = 6 WT, 5 KEP mice; Mann–Whitney U-test). All data are mean + s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Figure 4 Neutrophils influence the function and phenotype of CD8+ T cells.
a, Unsupervised hierarchical clustering of RNA-Seq analysis depicting 100 differentially expressed genes between circulating neutrophils from WT and tumour-bearing KEP mice. P value (0.05) was used as cutoff (n = 4 WT, 5 KEP mice). See also Extended Data Table 1 for top 50 genes ranked by fold change. b, Circulating neutrophils from either WT or tumour-bearing KEP mice were incubated with CFSE-labelled splenic CD8+ T cells from WT mice and CD3/CD28 stimulation beads. The iNOS inhibitor, L-NMMA, was added where indicated. After 48 h, CD8+ T cell proliferation was measured by flow cytometry. c, Dot plots depicting live cell-gated CD8+ T cell proportions in lungs of mice in control and neutrophil-depleted mice, killed when transplanted tumours reached 100 mm2. d, Dot plots of effector CD8+ T cell (CD62L−CD44+) proportions in lungs of transplanted mammary tumour-bearing mice that were killed when tumours reached 100 mm2. e, IFN-γ expression by CD8+ T cells in lungs of transplanted mammary tumour-bearing mice that were killed when tumours reached 100 mm2. f, Tumour growth kinetics in neutrophil-depleted or combined neutrophil- and CD8+ T-cell-depleted, mammary tumour-transplanted recipient mice, compared with control (n = 13 control, 21 anti-Ly6G, 14 anti-Ly6G/CD8). Data are mean + s.e.m.
Extended Data Figure 5 Cytokine expression levels in tumours and T cells, and their effects on neutrophils.
a, Unsupervised clustering of cytokine expression analysis in WT mammary glands and KEP tumours. Protein lysates were prepared as previously described from whole tissue34 and analysed for expression of various cytokines by Luminex-based assay (n = 5 per group). b, Protein levels of indicated cytokines in WT mammary glands and KEP tumours, determined by Luminex-based cytokine profiling; n.d., not detectable (n = 10 per group; Mann–Whitney U-test). c, d, Quantification of neutrophil and cKIT-expressing neutrophil accumulation in blood as determined by flow cytometry and gated on CD45+ cells. WT (n = 4) or tumour-bearing KEP mice (n = 9) were treated with anti-IL-17 (n = 8) and/or recombinant G-CSF (rG-CSF; n = 4) where indicated (Mann–Whitney U-test or Kruskal–Wallis test followed by post-hoc Dunn’s test). e, Gene expression in circulating neutrophils from WT control (n = 5), rG-CSF-treated WT mice (n = 4), KEP control (n = 10), anti-IL-17-treated (n = 6), anti-IL-17 + rG-CSF-treated KEP mice (n = 4; Mann–Whitney U-test or Kruskal–Wallis test followed by post-hoc Dunn’s test). f, Spleens of three WT mice and three KEP mice were pooled and CD3+ T cells were isolated. These cells were analysed by a real-time PCR array containing 86 different genes. Gene expression changes greater than threefold are shown. Members of the IL-17 signalling pathway are depicted in blue. *P < 0.05, **P < 0.01, ***P < 0.001. All data are mean + s.e.m.
Extended Data Figure 6 Absence of the adaptive immune system reduces metastasis.
a, Graphic representation of mammary tumour latency (left) and tumour growth (right) in lymphocyte-proficient KEP;Rag1+/− and lymphocyte-deficient KEP;Rag1−/− mice (n = 30 per group left panel, 10 mice per group right panel). b, Levels of TGF-β1 in mammary tumours and the plasma of tumour-bearing mice (n = 6 tumour, 3 plasma). c, Quantification of metastatic burden in lungs of recipient WT or Rag1−/− mice that were transplanted with KEP mammary tumour fragments and underwent surgical removal of the primary tumour (n = 6 WT, 4 Rag1−/− mice; **P < 0.01, Mann–Whitney U-test). Data are mean + s.e.m.
Extended Data Figure 7 Depletion of CD4+ T cells does not affect systemic cytokine levels or neutrophil expansion.
a, The proportion of IL-17A+ cells among CD4+ T cells in organs of WT and tumour-bearing KEP mice (n = 6 per group; Mann–Whitney U-test). b, Median fluorescence intensity of IL-17A expression in circulating γδ and CD4+ T cells from tumour-bearing KEP mice, as determined by flow cytometry (n = 11 per group; Wilcoxon matched-pairs test). c, Representative dot plots depicting total neutrophil and cKIT+ proportions in blood of control, anti-CD4- and anti-γδTCR-treated tumour-bearing KEP mice. d, Quantification of total neutrophil and cKIT+ neutrophil proportions in blood of control and anti-CD4-treated tumour-bearing KEP mice (n = 7 per group; Mann–Whitney U-test). e, Serum levels of IL-17A and G-CSF in control and anti-CD4-treated tumour-bearing KEP mice (n = 10 control, 6 anti-CD4; Mann–Whitney U-test). *P < 0.05, **P < 0.01. All data are mean + s.e.m.
Extended Data Figure 8 γδ T cell phenotype in KEP mice and their lack of influence on tumour growth in the metastasis model.
a, γδ T cells from lungs of tumour-bearing KEP mice were analysed by flow cytometry for IL-17, CD27, Vγ1 and Vγ4 expression. Two major populations of γδ T cells were observed including IL-17+CD27− and IL-17−CD27+ cells. b, Representative histograms of CCR6, IL-1R1, IL-23R and ROR-γt expression in IL-17+CD27− and IL-17−CD27+ γδ T cell populations shown in a. c, Il1β gene expression in various cell populations isolated from transplanted KEP tumours. Tumours from three mice were pooled to form one group. CD45− cells (which include cancer cells, endothelial cells and fibroblasts), CD45+CD11b+F4/80+ macrophages, CD45+CD11b+Ly6G+ neutrophils and CD45+CD11b− lymphocytes were sorted from two pooled groups. Real-time PCR was performed on individual cell populations for Il1β expression. Relative expression among different cells is shown. d, Graphic representation of immune cell proportions in KEP tumours (n = 4). e, Primary tumour growth kinetics of control and γδ T cell-depleted tumour transplant recipient mice (n = 13 per group). f, Growth kinetics of primary tumours transplanted into Tcrd+/− (n = 10) and Tcrd−/− mice (n = 6). All data are mean + s.e.m.
Extended Data Figure 9 The γδ T cell/IL-17/neutrophil axis promotes breast cancer metastasis.
Mammary tumours evoke a systemic inflammatory cascade that is initiated by IL-1β production. Tumour-derived IL-1β activates γδ T cells to produce IL-17. Increased systemic IL-17 levels lead to upregulation of G-CSF, which subsequently causes neutrophil expansion and alteration of neutrophil phenotype. These phenotypically altered neutrophils produce iNOS that suppresses the activity of anti-tumour CD8+ T cells. As a result of this systemic inflammatory cascade, the chance that disseminated cancer cells can establish metastases in distant organs is maximized.
Rights and permissions
About this article
Cite this article
Coffelt, S., Kersten, K., Doornebal, C. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015). https://doi.org/10.1038/nature14282
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14282
This article is cited by
-
Neutrophils in cancer: dual roles through intercellular interactions
Oncogene (2024)
-
LncRNA Malat1 suppresses pyroptosis and T cell-mediated killing of incipient metastatic cells
Nature Cancer (2024)
-
Association of clinical biomarkers and response to neoadjuvant therapy in breast cancer
Irish Journal of Medical Science (1971 -) (2024)
-
Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment
Molecular Cancer (2023)
-
Exploiting innate immunity for cancer immunotherapy
Molecular Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.