Abstract
Inflammation promotes regeneration of injured tissues through poorly understood mechanisms, some of which involve interleukin (IL)-6 family members, the expression of which is elevated in many diseases including inflammatory bowel diseases and colorectal cancer. Here we show in mice and human cells that gp130, a co-receptor for IL-6 cytokines, triggers activation of YAP and Notch, transcriptional regulators that control tissue growth and regeneration, independently of the gp130 effector STAT3. Through YAP and Notch, intestinal gp130 signalling stimulates epithelial cell proliferation, causes aberrant differentiation and confers resistance to mucosal erosion. gp130 associates with the related tyrosine kinases Src and Yes, which are activated on receptor engagement to phosphorylate YAP and induce its stabilization and nuclear translocation. This signalling module is strongly activated upon mucosal injury to promote healing and maintain barrier function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Primary accessions
ArrayExpress
Data deposits
Microarray data reported here have been deposited in the ArrayExpress database under accession E-MEXP-E-MTAB-2400.
References
Ben-Neriah, Y. & Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nature Immunol. 12, 715–723 (2011)
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008)
Baddour, J. A., Sousounis, K. & Tsonis, P. A. Organ repair and regeneration: an overview. Birth Defects Res. C 96, 1–29 (2012)
Johnson, R. & Halder, G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nature Rev. Drug Discov. 13, 63–79 (2014)
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010)
Neurath, M. F. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 7, 6–19 (2014)
Garbers, C. et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 23, 85–97 (2012)
Kishimoto, T. IL-6: from its discovery to clinical applications. Int. Immunol. 22, 347–352 (2010)
Yoshimura, A., Naka, T. & Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nature Rev. Immunol. 7, 454–465 (2007)
Putoczki, T. & Ernst, M. More than a sidekick: the IL-6 family cytokine IL-11 links inflammation to cancer. J. Leukoc. Biol. 88, 1109–1117 (2010)
Rose-John, S., Mitsuyama, K., Matsumoto, S., Thaiss, W. M. & Scheller, J. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr. Pharm. Des. 15, 2095–2103 (2009)
Pilati, C. et al. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J. Exp. Med. 208, 1359–1366 (2011)
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009)
Rebouissou, S. et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 457, 200–204 (2009)
Tanaka, T., Narazaki, M. & Kishimoto, T. Therapeutic targeting of the interleukin-6 receptor. Annu. Rev. Pharmacol. Toxicol. 52, 199–219 (2012)
Cai, J. et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 24, 2383–2388 (2010)
Okamoto, R. et al. Requirement of Notch activation during regeneration of the intestinal epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G23–G35 (2009)
Yu, F. X. & Guan, K. L. The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 (2013)
Rosenbluh, J. et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151, 1457–1473 (2012)
Bray, S. J. Notch signalling: a simple pathway becomes complex. Nature Rev. Mol. Cell Biol. 7, 678–689 (2006)
Madison, B. B. et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 (2002)
Camargo, F. D. et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 (2007)
Zhou, D. et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl Acad. Sci. USA 108, E1312–E1320 (2011)
Fre, S. et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435, 964–968 (2005)
van Es, J. H. et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005)
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013)
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011)
Murakami, D. et al. Presenilin-dependent gamma-secretase activity mediates the intramembranous cleavage of CD44. Oncogene 22, 1511–1516 (2003)
Zhang, J. et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nature Cell Biol. 11 1444–1450 10.1038/ncb1993 (2009)
Nishioka, N. et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 16, 398–410 (2009)
Chen, J., Elfiky, A., Han, M., Chen, C. & Saif, M. W. The role of Src in colon cancer and its therapeutic implications. Clin. Colorectal Cancer 13, 5–13 (2014)
Levy, D., Adamovich, Y., Reuven, N. & Shaul, Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol. Cell 29, 350–361 (2008)
Azzolin, L. et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170 (2014)
Sudol, M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene 9, 2145–2152 (1994)
Tsutsumi, R. et al. YAP and TAZ, Hippo signaling targets, act as a rheostat for nuclear SHP2 function. Dev. Cell 26, 658–665 (2013)
Tschaharganeh, D. F. et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology 144, 1530–1542 (2013)
Li, Y., Hibbs, M. A., Gard, A. L., Shylo, N. A. & Yun, K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells 30, 741–752 (2012)
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012)
Gordon, M. et al. The tumor suppressor gene, RASSF1A, is essential for protection against inflammation-induced injury. PLoS ONE 8, e75483 (2013)
Tamm, C., Bower, N. & Anneren, C. Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signaling pathway downstream of LIF. J. Cell Sci. 124, 1136–1144 (2011)
Raz, R., Lee, C. K., Cannizzaro, L. A., d’Eustachio, P. & Levy, D. E. Essential role of STAT3 for embryonic stem cell pluripotency. Proc. Natl Acad. Sci. USA 96, 2846–2851 (1999)
Taniguchi, K. & Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 26, 54–74 (2014)
Hedvat, M. et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 16, 487–497 (2009)
Cordero, J. B. et al. c-Src drives intestinal regeneration and transformation. EMBO J. 33, 1474–1491 (2014)
Takeda, K. et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49 (1999)
Zhang, N. et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010)
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nature Med. 15, 701–706 (2009)
Holzer, R. G. et al. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 147, 173–184 (2011)
Hu, H. T. et al. Tie2-R849W mutant in venous malformations chronically activates a functional STAT1 to modulate gene expression. J. Invest. Dermatol. 128 2325–2333 10.1038/jid.2008.89 (2008)
Nam, J. S., Ino, Y., Sakamoto, M. & Hirohashi, S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin. Cancer Res. 8, 2430–2436 (2002)
Lee, S. H. et al. ERK activation drives intestinal tumorigenesis in Apcmin/+ mice. Nature Med. 16, 665–670 (2010)
Hu, L., Zaloudek, C., Mills, G. B., Gray, J. & Jaffe, R. B. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin. Cancer Res. 6, 880–886 (2000)
Katakura, K. et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Invest. 115, 695–702 (2005)
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009)
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772 (2011)
Guma, M. et al. Constitutive intestinal NF-κB does not trigger destructive inflammation unless accompanied by MAPK activation. J. Exp. Med. 208, 1889–1900 (2011)
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007)
Umemura, A. et al. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metab. 20, 133–144 (2014)
Mitchell, C. & Willenbring, H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nature Protocols 3, 1167–1170 (2008)
Kozak, I. et al. A degenerative retinal process in HIV-associated non-infectious retinopathy. PLoS ONE 8, e74712 (2013)
Acknowledgements
We thank D. Pan and S. Akira for Yapfl/fl and Stat3fl/fl mice, respectively. We also thank D. L. Gumucio for a plasmid containing the 12.4-kb villin promoter, T. Sato, H. Clevers and Y. Hippo for protocols describing intestinal organoid culture, C. Kuo for R-spondin1-producing cells, D. Huszar for AZD1480, F. Schaper for plasmids, L. Eckmann for advice, A. Umemura, H. Nakagawa, H. Ogata, E. J. Park, G. Y. Yu, J. Font-Burgada, D. Dhar, J. Kim and E. Seki for providing liver samples, J. Zhao, T. Meerloo, Y. Jones, L. Gapuz, R. Ly, N. Varki, D. Aki, N. Hiramatsu, T. Moroishi, Y. Endo, H. Nishinakamura, A. Chang and T. Lee for technical advice and assistance, and Cell Signaling, Santa Cruz Biotechnology and GeneTex for antibodies. This work was supported by Postdoctoral Fellowship for Research Abroad and Research Fellowship for Young Scientists from the Japan Society for the Promotion of Science, a Uehara Memorial Foundation Fellowship, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Kanae Foundation for the Promotion of Medical Science to K.T.; a traveling grant NSC-101-2918-I-006-005 and a research grant NSC-103-2320-B-006-032 by National Science Council of Taiwan to L.-W.W.; NIH R00DK088589, FCCC-Temple University Nodal grant, AACR-Landon Innovator Award in Tumor Microenvironment, and the Pew Scholar in Biomedical Sciences Program for S.I.G.; a CCFA fellowship (RFA2927) to P.R.d.J.; Croucher Foundation and China Postdoctoral Science Foundation to K.W.; by the Research Service of the Department of Veterans Affairs to S.B.H.; by the NIH and the UCSD Digestive Disease Research Center Grant to J.T.C. and W.J.S.; by the NIH EY022611 and CA132809 to K.-L.G.; and by the NIH CA118165-09 and AACR to M.K., who is an American Cancer Society Research Professor and holds the Ben and Wanda Hildyard Chair for Mitochondrial and Metabolic Diseases.
Author information
Authors and Affiliations
Contributions
K.T. and M.K. conceived the project. K.T., L.-W.W., S.I.G., P.R.d.J., I.L., F.-X.Y., K.W., G.H. performed the experiments. K.T., L.-W.W., P.R.d.J, G.H. and M.K. analysed data. J.Z.-R. provided gp130 mutants, S.B.H., J.T.C., B.S.B. and W.J.S. provided human specimens, S.I.G., E.R., Y.M., A.Y., J.Z.-R. and K.-L.G. provided conceptual advice. K.T., L.-W.W. and M.K. wrote the manuscript, with all authors contributing to the writing and providing advice.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
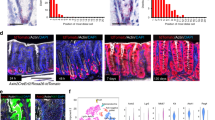
Extended Data Figure 1 gp130Act expression and intestinal phenotype.
a, Schematic diagram of the villin-gp130Act transgenic (Tg) construct and the gp130Act and gp130SA variants. b, Expression of gp130Act in the villin-gp130Act jejunum was confirmed by RT–PCR with specific primers for human gp130. Cyclophilin (CPH) was used as an internal control. c, Representative images of wild-type (WT) and villin-gp130Act intestines. c-Myc and cyclin D1 (d) and TUNEL (red, TUNEL; blue, DAPI) (e) staining of paraffin-embedded sections of wild-type and villin-gp130Act small intestines from 3-month-old mice. Positive cells were enumerated in each villus or crypt (n = 6). Data represent averages ± s.d.; P < 0.05. f, MMP7-, AB- and ChrA-positive cells in wild-type and villin-gp130Act small intestines were enumerated in each villus or crypt (n = 6). Data represent averages ± s.d.; P < 0.05. g, Paraffin-embedded sections of wild-type and villin-gp130Act small intestines were analysed by PAS and lysozyme staining. Positive cells were enumerated in each villus or crypt (n = 6). Data represent averages ± s.d.; P < 0.05. h, Cryptdin mRNA in wild-type and villin-gp130Act jejunum was detected by in situ hybridization. i, Transmission electron microscopy (TEM) of the apical surface of wild-type and transgenic small intestines. Scale bars represent 100 μm (d, e, g, h) and 1 μm (i) and all data are representative of at least 2–3 independent experiments.
Extended Data Figure 2 Aberrant intestinal differentiation and activation of gp130 effectors in gp130Act mice.
a, Paraffin-embedded sections of wild-type and villin-gp130Act (Tg) small intestines were analysed by CD45 staining. b, Lysates of wild-type and villin-gp130Act jejuna were prepared, and expression of Il6 and Tnf mRNAs was analysed by qRT–PCR (n = 3). Results are averages ± s.e.m.; P < 0.05. c, H&E and PAS staining of paraffin-embedded sections of wild-type and villin-gp130Act large intestines. d, pSTAT1, pERK1/2, pS6 and CD44 C-terminal stainings of paraffin-embedded sections of wild-type and villin-gp130Act small intestines. Positive cells were enumerated in each villus or crypt (n = 4). Data represent averages ± s.d.; P < 0.05. e, YAP, Ki67 and pSTAT3 stainings of paraffin-embedded sections of wild-type and villin-gp130Act large intestines. Positive cells were enumerated in each crypt (n = 4). Data represent averages ± s.d.; P < 0.05. Scale bars represent 100 μm (a, c-e) and all data are representative of at least 2–3 independent experiments.
Extended Data Figure 3 IL-6 and gp130 induce Notch and YAP activation in intestinal organoids and cancer cells, and gene expression analysis of intestinal crypts.
a, b, Wild-type and villin-gp130Act organoids were cultured, their RNA extracted, and expression of the indicated mRNA species was measured by qRT–PCR (n = 3). Results are averages ± s.e.m.; P < 0.05. c, Appearance of wild-type and villin-gp130Act small intestinal organoids cultured in standard EGF/Noggin/R-spondin 1 medium. d, Nuclei of T84 colon cancer cells transfected with either empty vector (EV) or a vector encoding superactive gp130 (gp130SA) were lysed and subjected to immunoblot analysis with the indicated antibodies. Lamin A, a nuclear marker. Actin, a loading control. e, Lysates of serum-starved SW480 (upper) or DLD1 (lower) colon cancer cells treated for 0–480 min with IL-6 at 50 ng ml−1 were subjected to immunoblot analysis using the indicated antibodies. f, Nuclei of serum-starved HT29 colon cancer cells treated for 24 h with IL-6 at 0–50 ng ml−1 were lysed and subjected to immunoblot analysis with the indicated antibodies. HDAC, a nuclear marker and loading control. g, Lysates of primary mouse hepatocytes treated for 0–120 min with IL-6 at 50 ng ml−1 were subjected to immunoblot analysis using the indicated antibodies. h, Microarray analysis was performed using the Illumina MouseWG-6 v2 Expression BeadChip on RNA extracted from wild-type and villin-gp130Act small intestinal crypts (n = 3 per group). Data were normalized and analysed as described and expression of the indicated genes is shown as fold-induction compared to wild-type crypts. i, RNA was extracted from wild-type and villin-gp130Act small intestinal organoids, and Areg mRNA expression was measured by qRT–PCR (n = 3). Results are averages ± s.e.m.; P < 0.05. Scale bars represent 100 μm (c) and all data are representative of at least 2–3 independent experiments.
Extended Data Figure 4 Aberrant intestinal differentiation in gp130Act mice depends on Notch and YAP but not on STAT3.
a, MMP7 staining of paraffin-embedded sections of control and DBZ-treated (10 μmol kg−1) villin-gp130Act small intestines. Positive cells were enumerated in each crypt (n = 3). Data represent averages ± s.d.; P < 0.05. b, MMP7 and lysozyme staining of paraffin-embedded sections of villin-gp130Act and villin-gp130Act/YapΔIEC small intestines. Positive cells were enumerated in each crypt (n = 4). Data represent averages ± s.d.; P < 0.05. c, PAS, Ki67, YAP, pSTAT3, HES1 and MMP7 staining of paraffin-embedded sections of villin-gp130Act and villin-gp130Act/Stat3ΔIEC small intestines. Positive cells were enumerated in each villus or crypt (n = 4). Data represent averages ± s.d.; P < 0.05. Scale bars represent 100 μm (a-c) and all data are representative of at least 2–3 independent experiments.
Extended Data Figure 5 MEK and PI(3)K inhibitors have no effect on aberrant intestinal homeostasis in gp130Act mice.
a, PAS, Ki67, YAP and pERK1/2 staining of paraffin-embedded sections of control and PD0325901-treated (25 mg kg−1) villin-gp130Act small intestines. Positive cells were enumerated in each villus or crypt (n = 3). Data represent averages ± s.d.; P < 0.05. b, PAS, Ki67, YAP and pS6 staining of paraffin-embedded sections of control and LY294002-treated (100 mg kg−1) villin-gp130Act small intestines. Positive cells were enumerated in each villus or crypt (n = 3). Data represent averages ± s.d.; P < 0.05. Scale bars represent 100 μm (a, b).
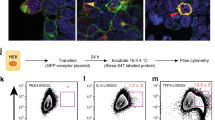
Extended Data Figure 6 gp130 activates YAP via a Hippo-independent but tyrosine phosphorylation-dependent mechanism, and gp130 interacts with Src and Yes.
a, Lysates of wild-type and villin-gp130Act jejuna, which are the same as the ones in Fig. 2a, were analysed for expression and phosphorylation of the indicated proteins. b, Lysates of HT29 colon cancer cells transfected with either empty vector (EV), active gp130 (gp130Act), or superactive gp130 (gp130SA) were subjected to immunoblot analysis with pSrc (Y419), total Src and GAPDH antibodies. GAPDH, a loading control. c, Lysates of HCA7 colon cancer cells infected with EV, wild-type gp130, gp130Act, or gp130SA lentiviruses were immunoprecipitated with anti-YAP antibody and blotted with the indicated antibodies. d, Lysates of HT29 colon cancer cells infected with EV, wild-type gp130, gp130Act, or gp130SA lentiviruses were immunoblot analysed for expression and phosphorylation of the indicated proteins. e, Serum-starved HCT116 cells were stimulated with 10% FBS, IL-6 (100 ng ml−1), or IL-11 (100 ng ml−1) for 30 min. Total cell lysates were analysed for expression and phosphorylation of the indicated proteins. f, Left: pSrc and YAP staining of livers from untreated wild-type mice (control) and wild-type mice 48 h after partial hepatectomy (PH). Scale bars represent 100 µm. Middle: lysates of livers from control mice and mice 48 h after partial hepatectomy were subjected to immunoblot analysis with the indicated antibodies. Right: lysates of livers from vehicle (DMSO)-treated and PP2-treated mice 48 h after partial hepatectomy were subjected to immunoblot analysis with the indicated antibodies. g, Top: HEK293T cells were transfected with plasmids expressing Flag–YAP. Twenty-four hours after transfection, the cells were pre-treated for 1 h with 0.1% DMSO (vehicle control), PP2 (10 μM) or AZD1480 (1 μM) and then were treated with 50 μg ml−1 cycloheximide for different time points. Total cell lysates were subjected to immunoblot analysis with the indicated antibodies. Bottom: HEK293T cells were transfected with Flag–YAP as above. Twenty-four hours after transfection, the cells were pre-treated for 1 h with 0.1% DMSO (vehicle control), AZD0530 (10 μM) or SU6656 (10 μM) and then were treated with 50 μg ml−1 cycloheximide for different time points. Total cell lysates were analysed as above. h, HEK293T cells were transfected with either empty or constitutively active (CA) Src expression vectors. After 48 h, the cells were lysed and expression of the indicated proteins determined by immunoblot analysis. i, HT29 cells were collected with or without 10 ng ml−1 IL-6 stimulation for 2 h. Lysates were analysed by immunoblot with the indicated antibodies. These are the loading controls for the data shown in Fig. 4e. j, HEK293T cells were transfected with expression vectors encoding Src and Flag-tagged gp130Act, Flag-tagged gp130Act (Δ771–811), Flag-tagged gp130Act (Δ812–827) or empty vector. Cells were collected 48 h later. Cell lysates were immunoprecipitated with Flag antibody and analysed by immunoblot with the indicated antibodies. k, Nuclei of T84 colon cancer cells infected with EV, gp130Act, gp130Act (Δ771–811) or gp130Act (Δ812–827) lentiviruses were prepared and subjected to immunoblot analysis with the indicated antibodies. HDAC1, a nuclear marker and loading control. All data are representative of at least 2–3 independent experiments.
Extended Data Figure 7 SFK activity is required for YAP activation.
a, Transgenic (Tg) mice (n = 4 per group) were treated with PP2 (5 mg kg−1) or vehicle once a day for 5 days. Small intestines were isolated, sectioned and stained as indicated. Positive cells were enumerated in each villus or crypt. Data represent averages ± s.d.; P < 0.05. b, c, Wild-type and villin-gp130Act small intestinal organoids were treated with DMSO, AZD0530 (10 μM) (b), AZD1480 (1 μM) or DBZ (10 μM) (c) for 24 h, stained with YAP antibody and counter stained with DAPI and photographed under a fluorescent microscope. d, Wild-type and villin-gp130Act small intestinal organoids were treated with DMSO, PP2 (10 μM) and AZD1480 (1 μM) for 24 h. Total cell lysates were subjected to immunoblot analysis with the indicated antibodies. e, Serum-starved HT29 cells were pre-treated for 1 h with 0.1% DMSO (vehicle control), AZD1480 (10 μM) or PP2 (20 μM) before IL-6 (10 ng ml−1) stimulation for 24 h. Nuclear extracts of HT29 cells treated without or with IL-6 in the absence or presence of AZD1480 or PP2 were subjected to immunoblot analysis with the indicated antibodies. Lamin A, a nuclear marker; Actin, a loading control. f, Wild-type and villin-gp130Act small intestinal organoids were treated with DMSO and AZD0530 (10 μM) for 24 h. Total cell lysates were subjected to immunoblot analysis with the indicated antibodies. Scale bars represent 100 μm (a–c). All data are representative of at least 2–3 independent experiments.
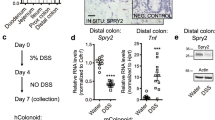
Extended Data Figure 8 gp130Act confers DSS resistance, induces Notch receptors and ligands and improves barrier function.
a, Left: representative images of wild-type and villin-gp130Act large intestines taken 10 days after 3.0% DSS treatment. Right: colon length of wild-type and villin-gp130Act mice before and after DSS treatment (before: n = 5 per group, after: n = 4 per group). Results are averages ± s.e.m.; P < 0.05. b, Representative images of H&E stained paraffin-embedded colon sections prepared 10 days after DSS challenge of wild-type and transgenic mice as described in Fig. 5a. Scale bars: 100 µm. c, Ki67 (left) and cleaved-caspase 3 (right) stainings were performed on paraffin-embedded colon sections from wild-type and transgenic mice at day 0 and 3 (Ki67) or 5 (cleaved-caspase 3) after 3.0% DSS treatment. d, Lysates of wild-type, villin-gp130Act, villin-gp130Act/YapΔIEC and villin-gp130Act/Stat3ΔIEC colons were prepared, RNA was extracted and expression of the indicated mRNA species was analysed by qRT–PCR (n = 3 per group). Results are averages ± s.e.m.; P < 0.05. e, f, Gut barrier function in wild-type and villin-gp130Act mice was examined by measurements of fecal albumin (WT, n = 6; Tg, n = 7) (e) and FITC-Dextran translocation to blood 4 h after oral gavage (WT, n = 5; Tg, n = 4) (f). Results are averages ± s.e.m.; P < 0.05. g, TEM images of intestinal mucosa epithelial cell–cell junctions in wild-type and villin-gp130Act small intestines. h, C57BL/6 mice were given regular water or 2.5% DSS for 7 days. Colonic RNA was extracted on day 10, and expression of the indicated genes was analysed by qRT–PCR (n = 4). Results are averages ± s.e.m.; P < 0.05. i, Wild-type mice were given 2.5% DSS. Colonic lysates were prepared when indicated and immunoblot analysed for protein expression and phosphorylation. j, Colon length of control and PP2-injected C57BL/6 mice after DSS treatment (n = 6 per group). Results are averages ± s.e.m.; P < 0.05. Scale bars represent 100 μm (b, c) and 500 nm (g).
Extended Data Figure 9 Enhanced mucosal regeneration in gp130Act mice depends on YAP and STAT3 but the two effectors control different genes, and YAP is required for in vitro scratch closure.
a, Left: body weight curves of DSS-treated YapΔIEC (squares, n = 6) and villin-gp130Act/YapΔIEC (circles, n = 4) mice. Results are averages ± s.d.; P < 0.05. Colon mucosal histology of YapΔIEC (squares, n = 6) and villin-gp130Act/YapΔIEC (circles, n = 4) mice was examined by H&E staining and scored 9 days after 2.0% DSS challenge by an observer blinded to the mouse genotype. Results are averages ± s.e.m.; P < 0.05. Right: body weight curves of DSS-treated Stat3ΔIEC (squares) and villin-gp130Act/Stat3ΔIEC (circles) mice (n = 4 per group). Results are averages ± s.d.; P < 0.05. Mucosal histology of Stat3ΔIEC and villin-gp130Act/Stat3ΔIEC mice (n = 4 per group) was examined and scored 8 days after 2.0% DSS challenge as above. Results are averages ± s.e.m.; P < 0.05. b, RNA was extracted from YapΔIEC and villin-gp130Act/YapΔIEC (n = 3 per group) or Stat3ΔIEC and villin-gp130Act/Stat3ΔIEC (n = 4 per group) colons, and expression of the indicated mRNA species was measured by qRT–PCR. Results are averages ± s.e.m.; P < 0.05. c, IEC6 rat intestinal epithelial cells infected with EV or gp130Act lentiviruses were grown to confluence and starved overnight, and the monolayers were wounded by scratching and treated with DMSO, PP2 (10 μM), AZD1480 (1 μM), verteporfin (1 μg ml−1) or DBZ (10 μM). The per cent wounded area was calculated by measuring wound closure over time (0 and 24 h). Results are averages ± s.e.m.; P < 0.05 (n = 5). d, Total cell lysates of IEC6 cells infected with EV + shluc (control), gp130Act + shluc or gp130Act + shYAP lentiviruses were prepared and subjected to immunoblot analysis with the indicated antibodies. e, IEC6 cells infected with EV + shluc (control), gp130Act + shluc or gp130Act + shYAP lentiviruses were grown to confluence and starved overnight, and the monolayers were wounded by scratching. The per cent wounded area was calculated by measuring wound closure over time (0 and 24 h) (n = 5). Results are averages ± s.e.m.; P < 0.05. f, Schematic representation of the gp130–SFK–YAP–Notch pathway and its function in the injured intestinal epithelium. Scale bars represent 100 μm (a).
Rights and permissions
About this article
Cite this article
Taniguchi, K., Wu, LW., Grivennikov, S. et al. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature 519, 57–62 (2015). https://doi.org/10.1038/nature14228
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14228
This article is cited by
-
The LKB1–TSSK1B axis controls YAP phosphorylation to regulate the Hippo–YAP pathway
Cell Death & Disease (2024)
-
Farnesoid X receptor promotes non-small cell lung cancer metastasis by activating Jak2/STAT3 signaling via transactivation of IL-6ST and IL-6 genes
Cell Death & Disease (2024)
-
VPS35 promotes gastric cancer progression through integrin/FAK/SRC signalling-mediated IL-6/STAT3 pathway activation in a YAP-dependent manner
Oncogene (2024)
-
IL-6/gp130 signaling: a key unlocking regeneration
Cell Regeneration (2023)
-
YAP 5-methylcytosine modification increases its mRNA stability and promotes the transcription of exosome secretion-related genes in lung adenocarcinoma
Cancer Gene Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.