Abstract
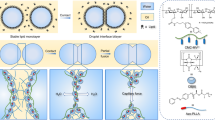
Emulsification is a powerful, well-known technique for mixing and dispersing immiscible components within a continuous liquid phase. Consequently, emulsions are central components of medicine, food and performance materials. Complex emulsions, including Janus droplets (that is, droplets with faces of differing chemistries) and multiple emulsions, are of increasing importance1 in pharmaceuticals and medical diagnostics2, in the fabrication of microparticles and capsules3,4,5 for food6, in chemical separations7, in cosmetics8, and in dynamic optics9. Because complex emulsion properties and functions are related to the droplet geometry and composition, the development of rapid, simple fabrication approaches allowing precise control over the droplets’ physical and chemical characteristics is critical. Significant advances in the fabrication of complex emulsions have been made using a number of procedures, ranging from large-scale, less precise techniques that give compositional heterogeneity using high-shear mixers and membranes10, to small-volume but more precise microfluidic methods11,12. However, such approaches have yet to create droplet morphologies that can be controllably altered after emulsification. Reconfigurable complex liquids potentially have great utility as dynamically tunable materials. Here we describe an approach to the one-step fabrication of three- and four-phase complex emulsions with highly controllable and reconfigurable morphologies. The fabrication makes use of the temperature-sensitive miscibility of hydrocarbon, silicone and fluorocarbon liquids, and is applied to both the microfluidic and the scalable batch production of complex droplets. We demonstrate that droplet geometries can be alternated between encapsulated and Janus configurations by varying the interfacial tensions using hydrocarbon and fluorinated surfactants including stimuli-responsive and cleavable surfactants. This yields a generalizable strategy for the fabrication of multiphase emulsions with controllably reconfigurable morphologies and the potential to create a wide range of responsive materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aserin, A. Multiple Emulsions: Technology and Applications (Wiley, 2008)
Gresham, P. A., Barnett, M. & Smith, S. V. Use of a sustained-release multiple emulsion to extend the period of radioprotection conferred by cysteamine. Nature 234, 149–150 (1971)
Lone, S. & Cheong, I. W. Fabrication of polymeric Janus particles by droplet microfluidics. RSC Adv. 4, 13322–13333 (2014)
Kim, J.-W., Utada, A. S., Fernández-Nieves, A., Hu, Z. & Weitz, D. A. Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angew. Chem. Int. Ed. 119, 1851–1854 (2007)
Shum, H. C. et al. Droplet microfluidics for fabrication of non-spherical particles. Macromol. Rapid Commun. 31, 108–118 (2010)
Augustin, M. A. & Hemar, Y. Nano-and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 38, 902–912 (2009)
Chakravarti, A., Chowdhury, S., Chakrabarty, S., Chakrabarty, T. & Mukherjee, D. Liquid membrane multiple emulsion process of chromium (VI) separation from waste waters. Colloids Surf. A 103, 59–71 (1995)
Patravale, V. & Mandawgade, S. Novel cosmetic delivery systems: an application update. Int. J. Cosmet. Sci. 30, 19–33 (2008)
Chen, L. et al. Photoresponsive monodisperse cholesteric liquid crystalline microshells for tunable omnidirectional lasing enabled by a visible light-driven chiral molecular switch. Adv. Optical Mater. 2, 845–848 (2014)
van der Graaf, S., Schroën, C. G. P. H. & Boom, R. M. Preparation of double emulsions by membrane emulsification—a review. J. Membr. Sci. 251, 7–15 (2005)
Shah, R. K. et al. Designer emulsions using microfluidics. Mater. Today 11, 18–27 (2008)
Choi, C.-H. et al. Microfluidic design of complex emulsions. ChemPhysChem 15, 21–29 (2014)
Zhao, C.-X. & Middelberg, A. P. J. Microfluidic mass-transfer control for the simple formation of complex multiple emulsions. Angew. Chem. Int. Ed. 48, 7208–7211 (2009)
Choi, C. H., Weitz, D. A. & Lee, C. S. One step formation of controllable complex emulsions: from functional particles to simultaneous encapsulation of hydrophilic and hydrophobic agents into desired position. Adv. Mater. 25, 2536–2541 (2013)
Haase, M. F. & Brujic, J. Tailoring of high-order multiple emulsions by the liquid–liquid phase separation of ternary mixtures. Angew. Chem. Int. Ed. 53, 11793–11797 (2014)
Song, Y. & Shum, H. C. Monodisperse w/w/w double emulsion induced by phase separation. Langmuir 28, 12054–12059 (2012)
Gladysz, J. A., Curran, D. P. & Horváth, I. T. Handbook of Fluorous Chemistry 18–20, 521–561 (Wiley-VCH, 2004)
Bedford, R. G. & Dunlap, R. D. Solubilities and volume changes attending mixing for the system: perfluoro-n-hexane-n-hexane. J. Am. Chem. Soc. 80, 282–285 (1958)
Wong, T.-S. et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 477, 443–447 (2011)
Duncanson, W. J. et al. Microfluidic fabrication of perfluorohexane-shelled double emulsions for controlled loading and acoustic-triggered release of hydrophilic agents. Langmuir 30, 13765–13770 (2014)
Guzowski, J., Korczyk, P. M., Jakiela, S. & Garstecki, P. The structure and stability of multiple micro-droplets. Soft Matter 8, 7269–7278 (2012)
Mukerjee, P. & Handa, T. Adsorption of fluorocarbon and hydrocarbon surfactants to air-water, hexane-water and perfluorohexane-water interfaces. Relative affinities and fluorocarbon-hydrocarbon nonideality effects. J. Phys. Chem. 85, 2298–2303 (1981)
McClain, B., Yoon, M., Litster, J. & Mochrie, S. Interfacial roughness in a near-critical binary fluid mixture: X-ray reflectivity and near-specular diffuse scattering. Eur. Phys. J. B 10, 45–52 (1999)
Brown, P., Butts, C. P. & Eastoe, J. Stimuli-responsive surfactants. Soft Matter 9, 2365–2374 (2013)
Rosen, M. J. & Kunjappu, J. T. Surfactants and Interfacial Phenomena 238 (Wiley, 2012)
Chevallier, E. et al. Pumping-out photo-surfactants from an air–water interface using light. Soft Matter 7, 7866–7874 (2011)
Li, X., Yang, Y., Eastoe, J. & Dong, J. Rich self-assembly behavior from a simple amphiphile. ChemPhysChem 11, 3074–3077 (2010)
Li, M., Powell, M. J., Razunguzwa, T. T. & O’Doherty, G. A. A general approach to anionic acid-labile surfactants with tunable properties. J. Org. Chem. 75, 6149–6153 (2010)
Rowlinson, J. S. & Widom, B. Molecular Theory of Capillarity 209–212 (Clarendon, 1982)
Yang, Y. et al. Environmentally responsive adsorption and assembly behaviors from N-alkyl-1,2-ethylenediamines. Soft Matter 9, 1458–1467 (2013)
Sletten, E. M. & Swager, T. M. Fluorofluorophores: fluorescent fluorous chemical tools spanning the visible spectrum. J. Am. Chem. Soc. 136, 13574–13577 (2014)
Haiying, L. & Zhongfan, L. A convenient synthesis of novel mercapto-ended azobenzene derivatives. Synth. Commun. 28, 3779–3785 (1998)
Hua, X. Y. & Rosen, M. J. Dynamic surface tension of aqueous surfactant solutions: 1. Basic parameters. J. Colloid Interface Sci. 124, 652–659 (1988)
Glantz, S. A. & Slinker, B. K. Primer of Applied Regression and Analysis of Variance 2nd edn, 248 (McGraw-Hill, 1990)
Harris, J. W. & Stöcker, H. Handbook of Mathematics and Computational Science 107 (Springer, 1998)
Canny, J. A computational approach to edge detection. IEEE Trans. Patt. Anal. Mach. Interf. PAMI-8, 679–698 (1986)
Taubin, G. Estimation of planar curves, surfaces, and nonplanar space curves defined by implicit equations with applications to edge and range image segmentation. IEEE Trans. Patt. Anal. Mach. Interf. 13, 1115–1138 (1991)
Fischer, P. & Erni, P. Emulsion drops in external flow fields — the role of liquid interfaces. Curr. Opin. Colloid Interface Sci. 12, 196–205 (2007)
Stone, H. A. & Leal, L. G. Breakup of concentric double emulsion droplets in linear flows. J. Fluid Mech. 211, 123–156 (1990)
Muguet, V. et al. W/O/W multiple emulsions submitted to a linear shear flow: correlation between fragmentation and release. J. Colloid Interface Sci. 218, 335–337 (1999)
Acknowledgements
Financial support from Eni S.p.A. under the Eni-MIT Alliance Solar Frontiers Program and by the US Army Research Laboratory and the US Army Research Office through the Institute for Soldier Nanotechnologies under contract number W911NF-13-D-0001 is acknowledged. E.M.S. and J.A.K. were supported by F32 Ruth L. Kirschtein NRSA Fellowships under award numbers EB014682 (E.M.S.) and GM106550 (J.A.K.). We thank L. Arriaga for introducing us to the fabrication of capillary microfluidic devices.
Author information
Authors and Affiliations
Contributions
L.D.Z. and T.M.S. developed the concept for the research. L.D.Z. conducted experiments involving emulsion fabrication and imaging, and measured interfacial tensions. V.S. and D.B. modelled the system and calculated and analysed equilibrium interfacial tensions. E.M.S. synthesized the fluorinated crosslinker and fluorinated coumarin dye. J.A.K. synthesized the light-responsive surfactant and the cleavable surfactant. All authors contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have filed two patent applications based on the research presented in this paper.
Extended data figures and tables
Extended Data Figure 1 Dynamic interfacial tension data was used to estimate the equilibrium interfacial tensions for the hexane–water and perfluorohexane–water interfaces.
a, Dynamic interfacial tension data (in blue) was obtained from the pendant-drop method; the representative data shown here was measured for the hexane–water interface at fSDS = 0.9 (such that the aqueous solution contained 0.1% SDS and 0.1% Zonyl in a 9:1 ratio). The data was fitted to an empirical model (in red) to estimate the equilibrium value of the interfacial tension γeqb = γ(t → ∞). Such fitting was performed for all measured interfacial tensions and the fitted parameter results are tabulated in Extended Data Table 1. b, The estimated equilibrium interfacial tension values were used to plot the hexane–water (squares) and perfluorohexane–water (circles) interfacial tensions as a function of the fraction of 0.1% SDS, fSDS, where the other fraction is 0.1% Zonyl. See discussion in Methods for more details.
Extended Data Figure 2 The geometry of a Janus droplet can be used to estimate the interfacial tension between hydrocarbon and fluorocarbon internal phases.
a, Sketch of a Janus droplet consisting of hydrocarbon (grey) and fluorocarbon (white) phases within an aqueous outer phase. The radii of curvature of the H–W (RH), F–W (RF) and F–H (RFH) interfaces are related to their respective interfacial tensions through the Young–Laplace equation. The diameter of the circle of contact between the two phases (dashed line) is denoted as D. b, The Janus droplet is composed of three spherical caps, and the volume, Vcap, of each constituent spherical cap is a function of the radius of curvature of the spherical surface and the base diameter D. Here we show the cap at the intersection of the hydrocarbon and fluorocarbon phases in which Vcap is a function of RFH and D. c, An exemplary image of a hexane–perfluorohexane Janus droplet obtained at fSDS = 0.6, which was used to estimate RFH and, in turn, γFH. d, The droplet pictured in c is subjected to edge detection to determine the H–W and F–W interfaces. e, The resulting edges are fitted to circles (red lines with green centres). The diameter of the circle of contact is then computed (green line). Given the ratio of the volumes of the two phases, we then determined the radius of curvature, RFH, of the hexane–perfluorohexane interface, which was subsequently used to estimate γFH. See discussion in Methods for more details and Extended Data Table 2 for estimated values of γFH.
Extended Data Figure 3 Reaction scheme and 1H-NMR of the fluorinated crosslinker.
a, Reaction scheme for the synthesis of the fluorinated crosslinker. BHT, 3,5-di-tert-butyl-4-hydroxytoluene. b, 1H-NMR spectrum of the fluorinated crosslinker.
Extended Data Figure 4 13C-NMR and 19F-NMR of the fluorinated crosslinker.
a, 13C-NMR spectrum of the fluorinated crosslinker. b, 19F-NMR of the fluorinated crosslinker.
Supplementary information
A drop of 10% Zonyl fluorosurfactant is added to a solution of 0.1% SDS-stabilized perfluorohexane/hexane/water double emulsions.
As Zonyl diffuses and creates a concentration gradient, we observe that the droplet morphology dynamically changes by first passing through a spherical Janus droplet conformation before inverting to a hexane/perfluorohexane/water double emulsion. The hexane phase is dyed red. The video is in real time. (MP4 15878 kb)
A drop of 5% SDS is added to a solution of 0.1% Zonyl-stabilized hexane/perfluorohexane/water double emulsions.
As SDS diffuses and creates a concentration gradient, we observe that the droplet morphology dynamically changes by first passing through a spherical Janus droplet conformation before inverting to a perfluorohexane/hexane/water double emulsion. The hexane phase is dyed red. The video is in real time. (MP4 10502 kb)
Droplets undergo a reversible transition from a hexane/perfluorohexane/water double emulsion to a Janus conformation in response to UV and blue light.
The transition is achieved by utilizing a combination of Zonyl fluorosurfactant and a light-responsive azobenzene surfactant. Most droplets are oriented with the denser perfluorohexane phase downward and are viewed from above, but a few droplets are pinned to the substrate and the transition is viewed from the side. The hexane phase is dyed red. The solution’s yellow tint results from the azobenzene surfactant used. The video is in real time. (MP4 16754 kb)
A droplet undergoes a reversible transition between perfluorohexane/hexane/water double emulsion and hexane/perfluorohexane/water double emulsion configurations.
The transition is achieved by utilizing a combination of Zonyl fluorosurfactant and a light-responsive azobenzene surfactant. The hexane phase is dyed red. The solution’s yellow tint results from the azobenzene surfactant used. The video is in real time. (MP4 4481 kb)
Rights and permissions
About this article
Cite this article
Zarzar, L., Sresht, V., Sletten, E. et al. Dynamically reconfigurable complex emulsions via tunable interfacial tensions. Nature 518, 520–524 (2015). https://doi.org/10.1038/nature14168
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14168
This article is cited by
-
Design automation of microfluidic single and double emulsion droplets with machine learning
Nature Communications (2024)
-
Nanostructure-free crescent-shaped microparticles as full-color reflective pigments
Nature Communications (2023)
-
Interfacial friction at action: Interactions, regulation, and applications
Friction (2023)
-
Responsive Janus droplets as modular sensory layers for the optical detection of bacteria
Analytical and Bioanalytical Chemistry (2023)
-
Breaking the photoswitch speed limit
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.