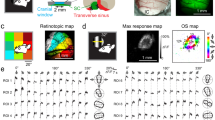
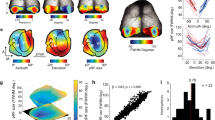
Abstract
More than twenty types of retinal ganglion cells conduct visual information from the eye to the rest of the brain1,2. Each retinal ganglion cell type tessellates the retina in a regular mosaic, so that every point in visual space is processed for visual primitives such as contrast and motion3. This information flows to two principal brain centres: the visual cortex and the superior colliculus. The superior colliculus plays an evolutionarily conserved role in visual behaviours4, but its functional architecture is poorly understood. Here we report on population recordings of visual responses from neurons in the mouse superior colliculus. Many neurons respond preferentially to lines of a certain orientation or movement axis. We show that cells with similar orientation preferences form large patches that span the vertical thickness of the retinorecipient layers. This organization is strikingly different from the randomly interspersed orientation preferences in the mouse’s visual cortex5; instead, it resembles the orientation columns observed in the visual cortices of large mammals6,7,8. Notably, adjacent superior colliculus orientation columns have only limited receptive field overlap. This is in contrast to the organization of visual cortex, where each point in the visual field activates neurons with all preferred orientations9. Instead, the superior colliculus favours specific contour orientations within ∼30° regions of the visual field, a finding with implications for behavioural responses mediated by this brain centre.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Masland, R. H. The neuronal organization of the retina. Neuron 76, 266–280 (2012)
Dhande, O. S. & Huberman, A. D. Retinal ganglion cell maps in the brain: implications for visual processing. Curr. Opin. Neurobiol. 24, 133–142 (2014)
Wässle, H. Parallel processing in the mammalian retina. Nature Rev. Neurosci. 5, 747–757 (2004)
May, P. J. The mammalian superior colliculus: laminar structure and connections. Prog. Brain Res. 151, 321–378 (2006)
Ohki, K., Chung, S., Ch’ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005)
Hubel, D. H. & Wiesel, T. N. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. (Lond.) 195, 215–243 (1968)
Blasdel, G. G. & Salama, G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature 321, 579–585 (1986)
Grinvald, A., Lieke, E., Frostig, R. D., Gilbert, C. D. & Wiesel, T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324, 361–364 (1986)
Harris, K. D. & Mrsic-Flogel, T. D. Cortical connectivity and sensory coding. Nature 503, 51–58 (2013)
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013)
Dräger, U. C. & Hubel, D. H. Topography of visual and somatosensory projections to mouse superior colliculus. J. Neurophysiol. 39, 91–101 (1976)
Dräger, U. C. & Hubel, D. H. Physiology of visual cells in mouse superior colliculus and correlation with somatosensory and auditory input. Nature 253, 203–204 (1975)
Wang, L. et al. Direction-specific disruption of subcortical visual behavior and receptive fields in mice lacking the β2 subunit of nicotinic acetylcholine receptor. J. Neurosci. 29, 12909–12918 (2009)
Wang, L., Sarnaik, R., Rangarajan, K., Liu, X. & Cang, J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J. Neurosci. 30, 16573–16584 (2010)
Zhao, X., Chen, H., Liu, X. & Cang, J. Orientation-selective responses in the mouse lateral geniculate nucleus. J. Neurosci. 33, 12751–12763 (2013)
Andermann, M. L., Kerlin, A. M., Roumis, D. K., Glickfeld, L. L. & Reid, R. C. Functional specialization of mouse higher visual cortical areas. Neuron 72, 1025–1039 (2011)
Swindale, N. V., Shoham, D., Grinvald, A., Bonhoeffer, T. & Hubener, M. Visual cortex maps are optimized for uniform coverage. Nature Neurosci. 3, 822–826 (2000)
Bosking, W. H., Crowley, J. C. & Fitzpatrick, D. Spatial coding of position and orientation in primary visual cortex. Nature Neurosci. 5, 874–882 (2002)
Yu, H., Farley, B. J., Jin, D. Z. & Sur, M. The coordinated mapping of visual space and response features in visual cortex. Neuron 47, 267–280 (2005)
Prusky, G. T. & Douglas, R. M. Characterization of mouse cortical spatial vision. Vision Res. 44, 3411–3418 (2004)
Huberman, A. D. et al. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron 62, 327–334 (2009)
Hong, Y. K., Kim, I. J. & Sanes, J. R. Stereotyped axonal arbors of retinal ganglion cell subsets in the mouse superior colliculus. J. Comp. Neurol. 519, 1691–1711 (2011)
Ramon-Moliner, E. Acetylthiocholinesterase distribution in the brain stem of the cat. Ergeb. Anat. Entwicklungsgesch. 46, 7–53 (1972)
Graybiel, A. M. A stereometric pattern of distribution of acetylthiocholinesterase in the deep layers of the superior colliculus. Nature 272, 539–541 (1978)
Chevalier, G. & Mana, S. Honeycomb-like structure of the intermediate layers of the rat superior colliculus, with additional observations in several other mammals: AChE patterning. J. Comp. Neurol. 419, 137–153 (2000)
Mana, S. & Chevalier, G. The fine organization of nigro-collicular channels with additional observations of their relationships with acetylcholinesterase in the rat. Neuroscience 106, 357–374 (2001)
Dean, P., Redgrave, P., Sahibzada, N. & Tsuji, K. Head and body movements produced by electrical stimulation of superior colliculus in rats: effects of interruption of crossed tectoreticulospinal pathway. Neuroscience 19, 367–380 (1986)
Sahibzada, N., Dean, P. & Redgrave, P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J. Neurosci. 6, 723–733 (1986)
Dean, P., Mitchell, I. J. & Redgrave, P. Responses resembling defensive behaviour produced by microinjection of glutamate into superior colliculus of rats. Neuroscience 24, 501–510 (1988)
Basole, A., White, L. E. & Fitzpatrick, D. Mapping multiple features in the population response of visual cortex. Nature 423, 986–990 (2003)
Mrsic-Flogel, T. D. et al. Altered map of visual space in the superior colliculus of mice lacking early retinal waves. J. Neurosci. 25, 6921–6928 (2005)
Cang, J., Wang, L., Stryker, M. P. & Feldheim, D. A. Roles of ephrin-as and structured activity in the development of functional maps in the superior colliculus. J. Neurosci. 28, 11015–11023 (2008)
Dorr, A., Sled, J. G. & Kabani, N. Three-dimensional cerebral vasculature of the CBA mouse brain: a magnetic resonance imaging and micro computed tomography study. Neuroimage 35, 1409–1423 (2007)
Wong, G. K., Poon, W. S., Yu, S. C. & Zhu, C. X. Transvenous embolization for dural transverse sinus fistulas with occluded sigmoid sinus. Acta Neurochirurgica 149, 929–935 (2007)
Dombeck, D. A., Khabbaz, A. N., Collman, F., Adelman, T. L. & Tank, D. W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56, 43–57 (2007)
Kaifosh, P., Lovett-Barron, M., Turi, G. F., Reardon, T. R. & Losonczy, A. Septo-hippocampal GABAergic signaling across multiple modalities in awake mice. Nature Neurosci. 16, 1182–1184 (2013)
Ohki, K. & Reid, R. C. In vivo two-photon calcium imaging in the visual system. Cold Spring Harb. Protoc. 2014, 402–416 (2014)
Göbel, W. & Helmchen, F. In vivo calcium imaging of neural network function. Physiology 22, 358–365 (2007)
Kerlin, A. M., Andermann, M. L., Berezovskii, V. K. & Reid, R. C. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67, 858–871 (2010)
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods 6, 875–881 (2009)
Acknowledgements
We thank E. Soucy and J. Greenwood for assistance with instrumentation; M. Joesch, A. Krishnaswamy, D. Kostadinov, S. Pashkovski, A. Giessel, T. Dunn, G. Keller, P. Kaifosh, M. Amoroso, and H. Asari for software; M. Andermann, V. Bonin, and F. Engert for advice on microscope design; J. Cohen for headplate designs; D. Anderson, K. Blum, B. Ölveckzy, and J. Sanes for critical reading of the manuscript; and J. Sanes for providing laboratory space and support to E.H.F. E.H.F. was supported by NIH T32 NS007484 and a Howard Hughes Medical Institute-Helen Hay Whitney Foundation fellowship. Additional support was provided by an NIH grant to M.M.
Author information
Authors and Affiliations
Contributions
E.H.F. designed the study, performed all experiments, interpreted results, and wrote the manuscript. M.M. helped design the study, interpret results, and write the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Plugs and head plates used for this study.
a, Top view of triangular silicone plug attached to a 5 mm coverslip. b, Side view of the plug from a. c, Suction cup used to position plug. d, Standard headplate with 8 mm aperture. e, Monitor tilt used to reduce fisheye distortion. Perspective is exaggerated for clarity. The monitor was tilted such that it was 20° from vertical, with the top nearer the animal, and 20° from the animal’s anterior–posterior axis, with the right edge closer to the animal. Note that stimuli were presented in a square area on the right side of the rectangular monitor. f, g, Azimuth and elevation in degrees for each pixel within the inscribed square area of the monitor on which stimuli were displayed. Values are given with respect to standard stereotaxic coordinates; because headplates were implanted at ∼15° angles with respect to this plane, bars on screen are tilted. Curvature of elevation bars reflects the fact that iso-elevation lines are curved, not straight, much like latitude lines on a globe.
Extended Data Figure 2 Orientation tuning does not reflect distortion effects of flat screen.
a, Plot of foot point of monitor (FP), the point at which a line from the eye is perpendicular to the tangent screen, and all mapped receptive field centres for orientation-tuned SC neurons in one animal. Each line segment is positioned at a cell’s receptive field centre and angled to reflect its preferred orientation. Orange line indicates the radial orientation relative to the FP for an example cell. Fisheye distortion will cause bars along the radial orientation relative to the foot point to appear relatively wider and faster than orthogonal bars along the tangential orientation, potentially biasing responses towards or away from the radial orientation. b, Enlarged view of inset area in a to show orientations more clearly. Note sharp transition in preferred orientation from bottom to top of panel relative to difference in radial orientation. Also note that the preferred orientation and the radial orientation vary with opposite handedness. c, Difference between radial orientation and preferred orientation for all cells in the plot. If the orientation map were due to fisheye distortion, preferred orientations should be similar to the radial orientation and the distribution should be centred at 0. Note that this distribution is biased away from 0 and centred between 45 and 90°. d–f, As in a–c for another animal. Note sharp transition in preferred orientations as in b, but with opposite handedness, and centring of distribution of preferred orientations between 0 and 45°. g–i, As in a–c for another animal. Note sharp transition in preferred orientations and bias of cells to orientations orthogonal to radial orientation. j–l, As in a–c for another animal. Note sharp transition in this field and a group of cells whose preferred orientations are close to radial.
Extended Data Figure 3 Sample responses of neurons in V1.
a, b, Average ΔF/F ± s.d. of two V1 neurons to 7 repetitions each of 8 directions of bar motion and a blank screen. Insets are polar plots for each cell.
Extended Data Figure 4 Monte Carlo simulation reveals significance of observed local similarity in the SC.
a, Absolute value of the difference in preferred orientations plotted against horizontal distance in the SC. Orange line indicates linear fit to the difference in preferred orientation as a function of horizontal separation, yielding a line of best fit with a slope of +22° per 100 µm. b, As in a after shuffling all cell positions. A total of 105 independent shuffles were performed; shown are results from the final shuffle. This yielded a distribution of slopes with a mean ± s.d. of (0 ± 1°) per 100 µm. c, Histogram of slopes of best-fit lines from 105 independent Monte Carlo simulations. Arrowhead indicates slope from a. d–f, As in a–c for data from V1.
Extended Data Figure 5 Orientation tuning of neuropil.
a, Schematic of cells and surrounding neuropil shell. Signals are extracted from each cell and from the neuropil within 20 µm. The corrected signal of a cell c within an ROI r is c = r – (f × n), with n the signal of the neuropil shell and f the fractional contamination by out-of-focus neuropil. b, Orientation preferences for neuropil shells of orientation-tuned cells in Fig. 2a. c, Difference in preferred orientation of neurons and their neuropil shells over a range of values of neuropil subtraction coefficient f. Dashed horizontal line indicates chance. Each black line reflects median values from a single image volume; orange line is median value for all cells from 7 volumes. d, Effects are robust over a range of f values. Plotted are mean differences in preferred orientations against distance ± s.e.m as in Fig. 2d, from which the orange trace is reproduced. Because the neuropil is also sharply tuned, using high values of f will reduce the apparent similarity of neighbouring cells’ orientation preferences. Nonetheless the similarity remains significant even at the excessively high f value of 0.9.
Extended Data Figure 6 Distribution of preferred orientations in the SC.
a, Preferred orientations of cells in the SC according to the presented orientation eliciting the strongest response. b, Preferred orientations of cells in the SC calculated from vector sums of responses to all orientations.
Extended Data Figure 7 Orientation maps do not reflect fisheye distortion.
a, Grating patches were presented at the foot point (FP), the point at which a line from the eye is perpendicular to the tangent screen, and at locations on the screen displaced from the FP along radial orientations of 0, 45, and 90°. b, Predicted map if fisheye distortion caused orientation tuning to be biased towards the radial orientation with respect to the FP. c, Projective field of foot point (black, indicated with white star) and patch at 0° (directly lateral on screen) from foot point (white patch, black star). d, As in c for patches at 90 and 45° with respect to foot point. e, Orientation map for this animal. Blue areas prefer horizontal bars, red areas prefer vertical bars, and arrows indicate lines from projective field of foot point to projective fields of patches. f, Expected orientation map according to distortion hypothesis. Note that the area at the projective field of the foot point should be untuned, and a line from the projective fields of the FP and a spot located at 0° relative elevation should pass from untuned areas to progressively more horizontal-preferring areas. Instead, it passes from a horizontal-preferring area at the FP to vertical-preferring areas as it moves to greater eccentricity. Trajectories along other projections of radial orientations (45 and 90°) are similarly poor fits to prediction. g, h, As in b and c for another animal. Orientation map for this animal in o also does not match prediction of fisheye distortion hypothesis. Arrow indicates shadow of blood vessel. i, j, Checkerboard pattern before and after ‘pre-distortion’ to offset fisheye effect. This pre-distortion was applied to change bar width by 1/cosine(θ), with θ the eccentricity from the FP, for both vertical and horizontal bar stimuli. k, l, Orientation maps for an animal in response to standard (k) and ‘pre-distorted’ (l) bar stimuli. In this animal the transverse sinus was not fully retracted and partially obscures the field of view. Note similarity of patterns in k and l. m, n, As in k, l, for the animal in c–e imaged on a different day. Comparison of maps in e and m reveals inter-trial variability, which is comparable to variability between standard and pre-distorted stimuli (m and n). o, p, As in k, l for a third animal. Map in o is overlaid with projective fields of points in visual field as in e. The reflectance change ΔR/R from black to white is 12 × 10−4 (c), 19 × 10−4 (d), and 18 × 10−4 (g, h). The reflectance change ΔR/R from red to blue is 4 × 10−4 (e, k, o), 5 × 10−4 (l, p), 7 × 10−4 (m), and 9 × 10−4 (n).
Extended Data Figure 8 Alternative mapping stimulus reveals similar projective fields.
a, Stimulus. Square grating patches alternate between two adjacent locations every 8 s. At each location the grating switches orientation randomly at 1 Hz. b, Map of responses elicited in animal from Fig. 4a. Responses to the two gratings span small patches on the surface of the SC. The reflectance change ΔR/R from black to white is 2 × 10−3. c, As in b for animal from Fig. 4c. The reflectance change ΔR/R from black to white is 2 × 10−3.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion. (PDF 91 kb)
Rights and permissions
About this article
Cite this article
Feinberg, E., Meister, M. Orientation columns in the mouse superior colliculus. Nature 519, 229–232 (2015). https://doi.org/10.1038/nature14103
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14103
This article is cited by
-
Rat superior colliculus encodes the transition between static and dynamic vision modes
Nature Communications (2024)
-
Topographic axonal projection at single-cell precision supports local retinotopy in the mouse superior colliculus
Nature Communications (2023)
-
Stimulus edges induce orientation tuning in superior colliculus
Nature Communications (2023)
-
High-density electrode recordings reveal strong and specific connections between retinal ganglion cells and midbrain neurons
Nature Communications (2022)
-
Short-Term Visual Experience Leads to Potentiation of Spontaneous Activity in Mouse Superior Colliculus
Neuroscience Bulletin (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.