Abstract
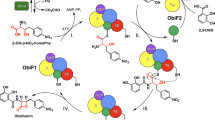
Non-ribosomal peptide synthetases are giant enzymes composed of modules that house repeated sets of functional domains, which select, activate and couple amino acids drawn from a pool of nearly 500 potential building blocks1. The structurally and stereochemically diverse peptides generated in this manner underlie the biosynthesis of a large sector of natural products. Many of their derived metabolites are bioactive such as the antibiotics vancomycin, bacitracin, daptomycin and the β-lactam-containing penicillins, cephalosporins and nocardicins. Penicillins and cephalosporins are synthesized from a classically derived non-ribosomal peptide synthetase tripeptide (from δ-(l-α-aminoadipyl)–l-cysteinyl–d-valine synthetase)2. Here we report an unprecedented non-ribosomal peptide synthetase activity that both assembles a serine-containing peptide and mediates its cyclization to the critical β-lactam ring of the nocardicin family of antibiotics. A histidine-rich condensation domain, which typically performs peptide bond formation during product assembly, also synthesizes the embedded four-membered ring. We propose a mechanism, and describe supporting experiments, that is distinct from the pathways that have evolved to the three other β-lactam antibiotic families: penicillin/cephalosporins, clavams and carbapenems. These findings raise the possibility that β-lactam rings can be regio- and stereospecifically integrated into engineered peptides for application as, for example, targeted protease inactivators3,4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
15 April 2015
A minor change was made to the main text final paragraph, and a present address added for author N.M.G.
References
Walsh, C. T., O'Brien, R. V. & Khosla, C. Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew. Chem. Int. Ed. 52, 7098–7124 (2013)
Banko, G., Demain, A. L. & Wolfe, S. δ-(l-α-Aminoadipyl)-l-cysteinyl-d-valine synthetase (ACV synthetase): a multifunctional enzyme with broad substrate specificity for the synthesis of penicillin and cephalosporin precursors. J. Am. Chem. Soc. 109, 2858–2860 (1987)
Buller, A. R. & Townsend, C. A. Intrinsic evolutionary constraints on protease structure, enzyme acylation, and the identity of the catalytic triad. Proc. Natl Acad. Sci. USA 110, E653–E661 (2013)
Galletti, P. & Giacomini, D. Monocyclic β-lactams: new structures for new biological activities. Curr. Med. Chem. 18, 4265–4283 (2011)
Hamad, B. The antibiotics market. Nature Rev. Drug Discov. 9, 675–676 (2010)
Demain, A. L. Antibiotics: natural products essential to human health. Med. Res. Rev. 29, 821–842 (2009)
McKenna, M. Antibiotic resistance: the last resort. Nature 499, 394–396 (2013)
Livermore, D. M. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 64, i29–i36 (2009)
Burzlaff, N. I. et al. The reaction cycle of isopenicillin N synthase observed by X-ray diffraction. Nature 401, 721–724 (1999)
Bachmann, B. O., Li, R. & Townsend, C. A. β-Lactam synthetase: a new biosynthetic enzyme. Proc. Natl Acad. Sci. USA 95, 9082–9086 (1998)
Freeman, M. F., Moshos, K. A., Bodner, M. J., Li, R. & Townsend, C. A. Four enzymes define the incorporation of coenzyme A in thienamycin biosynthesis. Proc. Natl Acad. Sci. USA 105, 11128–11133 (2008)
Davidsen, J. M. & Townsend, C. A. In vivo characterization of nonribosomal peptide synthetases NocA and NocB in the biosynthesis of nocardicin A. Chem. Biol. 19, 297–306 (2012)
Davidsen, J. M., Bartley, D. M. & Townsend, C. A. Non-ribosomal propeptide precursor in nocardicin A biosynthesis predicted from adenylation domain specificity dependent on the MbtH family protein NocI. J. Am. Chem. Soc. 135, 1749–1759 (2013)
Reeve, A. M., Breazeale, S. D. & Townsend, C. A. Purification, characterization, and cloning of an S-adenosylmethionine-dependent 3-amino-3-carboxypropyltransferase in nocardicin biosynthesis. J. Biol. Chem. 273, 30695–30703 (1998)
Townsend, C. A. & Brown, A. M. Nocardicin A: biosynthetic experiments with amino acid precursors. J. Am. Chem. Soc. 105, 913–918 (1983)
Hubbard, B. K., Thomas, M. G. & Walsh, C. T. Biosynthesis of l- p-hydroxyphenylglycine, a non-proteinogenic amino acid constituent of peptide antibiotics. Chem. Biol. 7, 931–942 (2000)
Townsend, C. A. & Wilson, B. A. The role of nocardicin G in nocardicin A biosynthesis. J. Am. Chem. Soc. 110, 3320–3321 (1988)
Gaudelli, N. M. & Townsend, C. A. Epimerization and substrate gating by a TE domain in β-lactam antibiotic biosynthesis. Nature Chem. Biol. 10, 251–258 (2014)
Walsh, C. T. et al. Tailoring enzymes that modify nonribosomal peptides during and after chain elongation on NRPS assembly lines. Curr. Opin. Struct. Biol. 5, 525–534 (2001)
Salituro, G. M. & Townsend, C. A. Total syntheses of (−)-nocardicins A–G: a biogenetic approach. J. Am. Chem. Soc. 112, 760–770 (1990)
Gunsior, M. et al. The biosynthetic gene cluster for a monocyclic β-lactam antibiotic, nocardicin A. Chem. Biol. 11, 927–938 (2004)
Belshaw, P. J., Walsh, C. T. & Stachelhaus, T. Aminoacyl-CoAs as probes of condensation domain selectivity in nonribosomal peptide synthesis. Science 284, 486–489 (1999)
Quadri, L. E. N. et al. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 37, 1585–1595 (1998)
Roche, E. D. & Walsh, C. T. Dissection of the EntF condensation domain boundary and active site residues in nonribosomal peptide synthesis. Biochemistry 42, 1334–1344 (2003)
Townsend, C. A., Brown, A. M. & Nguyen, L. T. Nocardicin A: stereochemical and biomimetic studies of monocyclic β-lactam formation. J. Am. Chem. Soc. 105, 919–927 (1983)
Samel, S. A., Schoenafinger, G., Knappe, T. A., Marahiel, M. A. & Essen, L.-O. Structural and functional insights into a peptide bond-forming bidomain from a nonribosomal peptide synthetase. Structure 15, 781–792 (2007)
Tahlan, K. & Jensen, S. E. Origins of the β-lactam rings in natural products. J. Antibiot. (Tokyo) 66, 401–410 (2013)
Sattely, E. S. & Walsh, C. T. A latent oxazoline electrophile for N-O-C bond formation in pseudomonine biosynthesis. J. Am. Chem. Soc. 130, 12282–12284 (2008)
Silverman, R. B. The Organic Chemistry of Enzyme-Catalyzed Reactions revised edn, 399–452 (Academic, 2002)
Arnison, P. G. et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160 (2013)
Ehmann, D. E., Trauger, J. W., Stachelhaus, T. & Walsh, C. T. Aminoacyl-SNACs as small-molecule substrates for the condensation domains of nonribosomal peptide synthetases. Chem. Biol. 7, 765–772 (2000)
Röttig, M. et al. NRPSpredictor2—a web server for predicting NRPS adenylation domain specificity. Nucleic Acids Res. 39, W362–W367 (2011)
Rausch, C., Weber, T., Kohlbacher, O., Wohlleben, W. & Huson, D. H. Specificity prediction of adenylation domains in nonribosomal peptide synthetases (NRPS) using transductive support vector machines (TSVMs). Nucleic Acids Res. 33, 5799–5808 (2005)
Clugston, S. L., Sieber, S. A., Marahiel, M. A. & Walsh, C. T. Chirality of peptide bond-forming condensation domains in nonribosomal peptide synthetases: the C5 domain of tyrocidine synthetase is a (D)C(L) catalyst. Biochemistry 42, 12095–12104 (2003)
Rausch, C., Hoof, I., Weber, T., Wohlleben, W. & Huson, D. H. Phylogenetic analysis of condensation domains in NRPS sheds light on their functional evolution. BMC Evol. Biol. 7, 78 (2007)
Acknowledgements
This work was supported by National Institutes of Health grant AI014937. We are indebted to D. W. Udwary (bioinformatics), K. A. Moshos, J. W. Labonte (chemistry) and R.-f. Li (molecular biology) for advice and discussion. We thank C. T. Walsh for the pET29-Sfp expression plasmid, I. P. Mortimer for high-resolution mass spectrometry (HRMS) data and C. Moore for help with NMR acquisitions.
Author information
Authors and Affiliations
Contributions
C.A.T. and N.M.G. developed the hypothesis and designed the study. N.M.G. and D.H.L. performed syntheses and biochemical experiments reported. All authors analysed and discussed the results. N.M.G., D.H.L. and C.A.T. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Mass spectrometric verification of apo to holo conversion of PCP4 with Sfp and l-pHPG-l-Arg-d-pHPG-l-Ser-S-coenzyme A (1), forming l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2).
Top: mass spectrum (ESI+) of apo-PCP4. Bottom: mass spectrum (ESI+) of l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2) derived from treatment of apo-PCP4 with Sfp and corresponding synthetic tetrapeptidyl-CoA substrate 1.
Extended Data Figure 2 Mass spectrum of major product generated from reaction catalysed by holo-module 5 supplemented with ATP, l-pHPG and l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2).
The major product observed from the reaction containing l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2), holo-module 5, ATP and l-pHPG was isolated over multiple injections by HPLC (peak isolated indicated in inset HPLC trace) and then characterized by HRMS (ESI+). The exact mass ion corresponding to the [M + H] ion of pro-nocardicin G was observed. Exact mass calculated for pro-nocardicin G: C33H39N8O9: 691.2835; found: 691.2839 [M + H]+.
Extended Data Figure 3 Negative experiments resulting from incubation of holo-module 5 with alternative substrates.
a, Schematic of incubation of the tetrapeptidyl substrate l-pHPG-l-Arg-d-pHPG-l-Ser-S-pantetheine (3) with holo-module 5 supplemented with ATP and l-pHPG. Pro-nocardicin G was not produced. b, HPLC traces of products obtained after incubation of l-pHPG-l-Arg-d-pHPG-l-Ser-S-pantetheine (3) and holo-module 5 (+M5(WT)) supplemented with ATP and l-pHPG. Pro-nocardicin G was not observed as verified by comparison with authentic standard. Additionally, it was noted that substrate 3 was not consumed over the duration of the experiment. (i) HPLC trace of the unbound products resulting from incubation of wild-type holo-module 5 reaction with l-pHPG-l-Arg-d-pHPG-l-Ser-S-pantetheine (3), l-pHPG and ATP. (ii) HPLC trace of the unbound products resulting from incubation of holo-M5l-pHPG-l-Arg-d-pHPG-l-Ser-S-pantetheine (3), l-pHPG and ATP. (iii) HPLC trace of incubation of l-pHPG-l-Arg-d-pHPG-l-Ser-S-pantetheine (3), l-pHPG and ATP without enzyme. The peak corresponding to l-pHPG-l-Arg-d-pHPG-l-Ser-S-pantetheine (3) substrate is indicated. (iv) HPLC trace of authentic standard of pro-nocardicin G. c, Schematic of incubation of the dipeptidyl substrate d-pHPG-l-Ser-S-PCP4 (5) with holo-module 5, l-pHPG and ATP. Nocardicin G, the corresponding expected product, was not observed. d, LC–MS traces of products obtained after incubation of d-pHPG-l-Ser-S-PCP4 (5) and holo-module 5 (+M5(WT)) supplemented with ATP and l-pHPG. The corresponding β-lactam product was not observed, as verified by comparison with authentic standard. (i) Total ion chromatogram of the unbound products resulting from wild-type holo-module 5 reaction with d-pHPG-l-Ser-S-PCP4 (5), l-pHPG and ATP. (ii) Extracted ion chromatogram of the wild-type reaction in trace (i), the 386.1 m/z ion, corresponding to [M + H] of nocardicin G was not observed. (iii) Total ion chromatogram of unbound products from holo-M5d-pHPG-l-Ser-S-PCP4 (5), l-pHPG and ATP. (iv) Extracted ion chromatogram of the wild-type reaction in trace (iii). (v) Extracted ion chromatogram of authentic standard of nocardicin G.
Extended Data Figure 4 Mass spectrometric comparison of apo with holo conversion of PCP4 with Sfp and d-pHPG-l-Ser-S-coenzyme A (4) forming d-pHPG-l-Ser-S-PCP4 (5).
Top: mass spectrum (ESI+) of apo-PCP4. Bottom: mass spectrum (ESI+) of d-pHPG-l-Ser-S-PCP4 (5) derived from treatment of apo-PCP4 with Sfp and corresponding synthetic dipeptidyl-CoA substrate 4. Owing to diketipiperazine22,31 formation and slow hydrolysis to the unloaded holo-PCP4, the half-life of 5 is approximately 20 min.
Extended Data Figure 5 Multiple sequence alignment of homologous NRPS C domains and the NocB C5 domain.
The alignment was performed using Clustal2 with gap opening and extending penalties of 10 and 0.1 respectively. The alignment included the N-terminal C fragment of EntF, the bacillibactin synthetase DhbF, the tyrocidine synthetase TycB, the surfactin synthetase SrfAC and the naturally free-standing condensation enzyme VibH. Highlighted in a red box is H790 uniquely present in the NocB C5 domain along with the corresponding residues present in the depicted C domains. The NocB C5 domain is N-terminal to the consensus catalytic motif, HHxxxDG, present in all canonical condensation domains.
Extended Data Figure 6 HPLC comparative analysis of the reactions catalysed by holo-module 5 and holo-M5l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2).
a, Schematic of incubation of the tetrapeptidyl substrate l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2) with holo-M5l-pHPG. Pro-nocardicin G was not produced. b, HPLC comparison of unbound products from reaction mixtures containing l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2) and either holo-module 5 or M5l-pHPG, indicating the formation of β-lactam product only from the wild-type reaction. (i) HPLC trace of the unbound products resulting from incubation of wild-type holo-module 5 reaction with l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2), l-pHPG and ATP. (ii) HPLC trace of the unbound products resulting from incubation of holo-M5l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2), l-pHPG and ATP. Pro-nocardicin G was not observed. (iii) HPLC trace of the unbound products resulting from incubation of holo-M5l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2), l-pHPG and ATP. (iv) HPLC trace of authentic standard of pro-nocardicin G.
Extended Data Figure 7 Mass spectral comparison of apo with holo conversion of PCP4 with Sfp and l-pHPG-l-Arg-d-pHPG-dehydroalanyl- S-3′-dephospho coenzyme A (6) forming l-pHPG-l-Arg-d-pHPG-dehydroalanyl- S-PCP4 (7).
Top: mass spectrum (ESI+) of apo-PCP4. Bottom: mass spectrum (ESI+) of l-pHPG-l-Arg-d-pHPG-dehydroalanyl- S-PCP4 (7) derived from treatment of apo-PCP4 with Sfp and corresponding synthetic eliminated tetrapeptidyl-CoA substrate 6.
Extended Data Figure 8 Mass spectrum of major product generated from reaction catalysed by holo-module 5 and l-pHPG-l-Arg-d-pHPG-dehydroalanyl- S-PCP4 (7) supplemented with ATP and l-pHPG.
The major product from the reaction containing l-pHPG-l-Arg-d-pHPG-dehydroalanyl- S-PCP4 (7) was isolated over multiple injections by HPLC (peak indicated in inset HPLC trace) and then determined by HRMS (ESI+). The exact mass ion corresponding to the [M + H]+ ion of pro-nocardicin G was observed. Exact mass calculated for C33H39N8O9: 691.2835; found: 691.2822 [M + H]+.
Extended Data Figure 9 Tandem mass spectrometric comparison of holo-module 5 synthesized pro-nocardicin G from l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2) and l-pHPG-l-Arg-d-pHPG-dehydroalanyl- S-PCP4 (7).
Fragmentation pattern of holo-module 5 biosynthetic pro-nocardicin G from serine-containing tetrapeptide substrate l-pHPG-l-Arg-d-pHPG-l-Ser-S-PCP4 (2, bottom) is directly comparable to the tandem mass spectrometric fragmentation pattern of holo-module 5 synthesized pro-nocardicin G from the dehydroalanine-containing substrate l-pHPG-l-Arg-d-pHPG-dehydroalanyl- S-PCP4 (7, middle). For comparison, the mass spectrum of synthetic pro-nocardicin G is shown at top.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Synthesis of Substrates, 1H-NMR Spectra of Substrates 1, 3, 4 and 6, Supplementary Tables 1 & 2 and additional references. (PDF 1273 kb)
Rights and permissions
About this article
Cite this article
Gaudelli, N., Long, D. & Townsend, C. β-Lactam formation by a non-ribosomal peptide synthetase during antibiotic biosynthesis. Nature 520, 383–387 (2015). https://doi.org/10.1038/nature14100
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14100
This article is cited by
-
Aminoacyl chain translocation catalysed by a type II thioesterase domain in an unusual non-ribosomal peptide synthetase
Nature Communications (2022)
-
Enzymatic assembly of the salinosporamide γ-lactam-β-lactone anticancer warhead
Nature Chemical Biology (2022)
-
Structures of a non-ribosomal peptide synthetase condensation domain suggest the basis of substrate selectivity
Nature Communications (2021)
-
Caerulomycin and collismycin antibiotics share a trans-acting flavoprotein-dependent assembly line for 2,2’-bipyridine formation
Nature Communications (2021)
-
Assessment of Synthesis Machinery of Two Antimicrobial Peptides from Paenibacillus alvei NP75
Probiotics and Antimicrobial Proteins (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.