Abstract
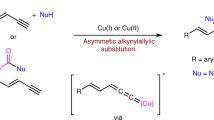
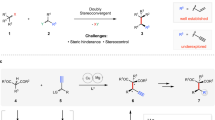
The development of new reactions forming asymmetric carbon–carbon bonds has enabled chemists to synthesize a broad range of important carbon-containing molecules, including pharmaceutical agents, fragrances and polymers1. Most strategies to obtain enantiomerically enriched molecules rely on either generating new stereogenic centres from prochiral substrates or resolving racemic mixtures of enantiomers. An alternative strategy—dynamic kinetic asymmetric transformation—involves the transformation of a racemic starting material into a single enantiomer product, with greater than 50 per cent maximum yield2,3. The use of stabilized nucleophiles (pKa < 25, where Ka is the acid dissociation constant) in palladium-catalysed asymmetric allylic alkylation reactions has proved to be extremely versatile in these processes4,5. Conversely, the use of non-stabilized nucleophiles in such reactions is difficult and remains a key challenge6,7,8,9. Here we report a copper-catalysed dynamic kinetic asymmetric transformation using racemic substrates and alkyl nucleophiles. These nucleophiles have a pKa of ≥50, more than 25 orders of magnitude more basic than the nucleophiles that are typically used in such transformations. Organometallic reagents are generated in situ from alkenes by hydrometallation and give highly enantioenriched products under mild reaction conditions. The method is used to synthesize natural products that possess activity against tuberculosis and leprosy, and an inhibitor of para-aminobenzoate biosynthesis. Mechanistic studies indicate that the reaction proceeds through a rapidly isomerizing intermediate. We anticipate that this approach will be a valuable complement to existing asymmetric catalytic methods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (eds) Comprehensive Asymmetric Catalysis: Suppl. 2 (Springer, 2004)
Huerta, F. F., Minidis, A. B. E. & Bäckvall, J. E. Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem. Soc. Rev. 30, 321–331 (2001)
Vedejs, E. & Jure, M. Efficiency in nonenzymatic kinetic resolution. Angew. Chem. Int. Edn 44, 3974–4001 (2005)
Trost, B. M. & VanVranken, D. L. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 96, 395–422 (1996)
Trost, B. M. & Fandrick, D. R. Palladium-catalyzed dynamic kinetic asymmetric allylic alkylation with the DPPBA ligands. Aldrichim. Acta 40, 59–72 (2007)
Pfaltz, A. & Lautens, M. in Comprehensive Asymmetric Catalysis ii Vol. 2 (eds Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. ) Ch. 24 833–884 (Springer, 1999)
Trost, B. M. & Thaisrivongs, D. A. Strategy for employing unstabilized nucleophiles in palladium-catalyzed asymmetric allylic alkylations. J. Am. Chem. Soc. 130, 14092–14093 (2008)
Sha, S. C., Zhang, J. D., Carroll, P. J. & Walsh, P. J. Raising the pKa limit of “soft” nucleophiles in palladium-catalyzed allylic substitutions: application of diarylmethane pronucleophiles. J. Am. Chem. Soc. 135, 17602–17609 (2013)
Lu, Z. & Ma, S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew. Chem. Int. Edn 47, 258–297 (2008)
Geurts, K., Fletcher, S. P., van Zijl, A. W., Minnaard, A. J. & Feringa, B. L. Copper-catalyzed asymmetric allylic substitution reactions with organozinc and Grignard reagents. Pure Appl. Chem. 80, 1025–1037 (2008)
Teichert, J. F. & Feringa, B. L. Phosphoramidites: privileged ligands in asymmetric catalysis. Angew. Chem. Int. Edn 49, 2486–2528 (2010)
Trost, B. M. & Bunt, R. C. Asymmetric induction in allylic alkylations of 3-(acyloxy)cycloalkenes. J. Am. Chem. Soc. 116, 4089–4090 (1994)
Misale, A., Niyomchon, S., Luparia, M. & Maulide, N. Asymmetric palladium-catalyzed allylic alkylation using dialkylzinc reagents: a remarkable ligand effect. Angew. Chem. Int. Edn 53, 7068–7073 (2014)
Trost, B. M. & Verhoeven, T. R. Allylic substitutions with retention of stereochemistry. J. Org. Chem. 41, 3215–3216 (1976)
Matsushita, H. & Negishi, E. Anti-stereospecificity in the palladium-catalyzed reactions of alkenyl-metal or aryl-metal derivatives with allylic electrophiles. Chem. Commun. 160–161 (1982)
Harutyunyan, S. R., den Hartog, T., Geurts, K., Minnaard, A. J. & Feringa, B. L. Catalytic asymmetric conjugate addition and allylic alkylation with Grignard reagents. Chem. Rev. 108, 2824–2852 (2008)
Alexakis, A., Bäckvall, J. E., Krause, N., Pamies, O. & Dieguez, M. Enantioselective copper-catalyzed conjugate addition and allylic substitution reactions. Chem. Rev. 108, 2796–2823 (2008)
Langlois, J. B. & Alexakis, A. in Topics in Organometallic Chemistry Vol. 38, Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis (ed. Kazmaier, U. ) 235–268 (Springer, 2012)
Norinder, J. & Bäckvall, J. E. Dynamic processes in the copper-catalyzed substitution of chiral allylic acetates leading to loss of chiral information. Chem. Eur. J. 13, 4094–4102 (2007)
Langlois, J. B. & Alexakis, A. Dynamic kinetic asymmetric transformation in copper catalyzed allylic alkylation. Chem. Commun. 3868–3870 (2009)
Langlois, J. B., Emery, D., Mareda, J. & Alexakis, A. Mechanistic identification and improvement of a direct enantioconvergent transformation in copper-catalyzed asymmetric allylic alkylation. Chem. Sci. 3, 1062–1069 (2012)
Giacomina, F. & Alexakis, A. Construction of enantioenriched cyclic compounds by asymmetric allylic alkylation and ring-closing metathesis. Eur. J. Org. Chem. 2013, 6710–6721 (2013)
Maksymowicz, R. M., Roth, P. M. C. & Fletcher, S. P. Catalytic asymmetric carbon-carbon bond formation using alkenes as alkylmetal equivalents. Nature Chem. 4, 649–654 (2012)
Sidera, M., Roth, P. M. C., Maksymowicz, R. M. & Fletcher, S. P. Formation of quaternary centers by copper-catalyzed asymmetric conjugate addition of alkylzirconium reagents. Angew. Chem. Int. Edn 52, 7995–7999 (2013)
Seemann, M., Schöller, M., Kudis, S. & Helmchen, G. Syntheses of enantiomerically pure cyclopent-2-ene-1-carboxylic acid and (cyclopent-2-enyl)acetic acid by enantioselective palladium-catalyzed allylic alkylations — synthesis of enantiomerically pure (-)-chaulmoogric acid. Eur. J. Org. Chem. 2122–2127 (2003)
Jacobsen, P. L. & Levy, L. Mechanism by which hydnocarpic acid inhibits mycobacterial multiplication. Antimicrob. Agents Chemother. 3, 373–379 (1973)
Cabot, M. C. & Goucher, C. R. Chaulmoogric acid-assimilation into the complex lipids of mycobacteria. Lipids 16, 146–148 (1981)
Wang, J. F. et al. Antituberculosis agents and an inhibitor of the para-aminobenzoic acid biosynthetic pathway from Hydnocarpus anthelminthica seeds. Chem. Biodivers. 7, 2046–2053 (2010)
Streitwieser, A., Jayasree, E. G., Hasanayn, F. & Leung, S. S. H. A theoretical study of SN2′ reactions of allylic halides: role of ion pairs. J. Org. Chem. 73, 9426–9434 (2008)
Zhang, H. & Gschwind, R. M. Structure identification of precatalytic copper phosphoramidite complexes in solution. Angew. Chem. Int. Edn 45, 6391–6394 (2006)
Acknowledgements
We acknowledge financial support from the EPSRC (EP/H003711/1, a Career Acceleration Fellowship to S.P.F.). B. Odell and T. Claridge are thanked for assistance with the NMR experiments.
Author information
Authors and Affiliations
Contributions
H.Y., E.R. and M.S. performed the experiments. All authors contributed to designing, analysing and discussing the experiments; S.P.F. conceived the work and guided the research. S.P.F. wrote the manuscript with assistance from H.Y. All authors contributed to discussing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors are all named as inventors on a UK patent application filed by Isis Innovation, which is the technology transfer arm of the University of Oxford.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data – see contents page for details. (PDF 37426 kb)
Rights and permissions
About this article
Cite this article
You, H., Rideau, E., Sidera, M. et al. Non-stabilized nucleophiles in Cu-catalysed dynamic kinetic asymmetric allylic alkylation. Nature 517, 351–355 (2015). https://doi.org/10.1038/nature14089
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14089
This article is cited by
-
Migratory allylic arylation of 1,n-enols enabled by nickel catalysis
Nature Communications (2023)
-
Mechanistic investigation of Rh(i)-catalysed asymmetric Suzuki–Miyaura coupling with racemic allyl halides
Nature Catalysis (2021)
-
Desymmetrization of meso-bisphosphates using copper catalysis and alkylzirconocene nucleophiles
Nature Communications (2019)
-
The organocatalytic highly enantioselective Knoevenagel condensation: applications in the synthesis of various chiral amide derivatives
Journal of the Iranian Chemical Society (2019)
-
Asymmetric Suzuki-Miyaura coupling of heterocycles via Rhodium-catalysed allylic arylation of racemates
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.