Abstract
Endocytosis is required for internalization of micronutrients and turnover of membrane components. Endophilin has been assigned as a component of clathrin-mediated endocytosis. Here we show in mammalian cells that endophilin marks and controls a fast-acting tubulovesicular endocytic pathway that is independent of AP2 and clathrin, activated upon ligand binding to cargo receptors, inhibited by inhibitors of dynamin, Rac, phosphatidylinositol-3-OH kinase, PAK1 and actin polymerization, and activated upon Cdc42 inhibition. This pathway is prominent at the leading edges of cells where phosphatidylinositol-3,4-bisphosphate—produced by the dephosphorylation of phosphatidylinositol-3,4,5-triphosphate by SHIP1 and SHIP2—recruits lamellipodin, which in turn engages endophilin. This pathway mediates the ligand-triggered uptake of several G-protein-coupled receptors such as α2a- and β1-adrenergic, dopaminergic D3 and D4 receptors and muscarinic acetylcholine receptor 4, the receptor tyrosine kinases EGFR, HGFR, VEGFR, PDGFR, NGFR and IGF1R, as well as interleukin-2 receptor. We call this new endocytic route fast endophilin-mediated endocytosis (FEME).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ringstad, N., Nemoto, Y. & De Camilli, P. The SH3p4/Sh3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc. Natl Acad. Sci. USA 94, 8569–8574 (1997)
Guichet, A. et al. Essential role of endophilin A in synaptic vesicle budding at the Drosophila neuromuscular junction. EMBO J. 21, 1661–1672 (2002)
Verstreken, P. et al. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell 109, 101–112 (2002)
Schuske, K. R. et al. Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron 40, 749–762 (2003)
Milosevic, I. et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron 72, 587–601 (2011)
Ringstad, N. et al. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron 24, 143–154 (1999)
Gad, H. et al. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron 27, 301–312 (2000)
Andersson, F., Low, P. & Brodin, L. Selective perturbation of the BAR domain of endophilin impairs synaptic vesicle endocytosis. Synapse 64, 556–560 (2010)
Sundborger, A. et al. An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. J. Cell Sci. 124, 133–143 (2011)
Perera, R. M., Zoncu, R., Lucast, L., De Camilli, P. & Toomre, D. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc. Natl Acad. Sci. USA 103, 19332–19337 (2006)
Ferguson, S. M. et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev. Cell 17, 811–822 (2009)
Taylor, M. J., Perrais, D. & Merrifield, C. J. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 9, e1000604 (2011)
Meinecke, M. et al. Cooperative recruitment of dynamin and BIN/amphiphysin/Rvs (BAR) domain-containing proteins leads to GTP-dependent membrane scission. J. Biol. Chem. 288, 6651–6661 (2013)
Jockusch, W. J., Praefcke, G. J., McMahon, H. T. & Lagnado, L. Clathrin-dependent and clathrin-independent retrieval of synaptic vesicles in retinal bipolar cells. Neuron 46, 869–878 (2005)
Llobet, A. et al. Endophilin drives the fast mode of vesicle retrieval in a ribbon synapse. J. Neurosci. 31, 8512–8519 (2011)
Tang, Y. et al. Identification of the endophilins (SH3p4/p8/p13) as novel binding partners for the beta1-adrenergic receptor. Proc. Natl Acad. Sci. USA 96, 12559–12564 (1999)
Petrelli, A. et al. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 416, 187–190 (2002)
Soubeyran, P., Kowanetz, K., Szymkiewicz, I., Langdon, W. Y. & Dikic, I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416, 183–187 (2002)
Cremona, O. et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99, 179–188 (1999)
Parton, R. G. & del Pozo, M. A. Caveolae as plasma membrane sensors, protectors and organizers. Nature Rev. Mol. Cell Biol. 14, 98–112 (2013)
Glebov, O. O., Bright, N. A. & Nichols, B. J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nature Cell Biol. 8, 46–54 (2006)
Lundmark, R. et al. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr. Biol. 18, 1802–1808 (2008)
Sigismund, S. et al. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell 15, 209–219 (2008)
Lamaze, C. et al. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell 7, 661–671 (2001)
Sauvonnet, N., Dujeancourt, A. & Dautry-Varsat, A. Cortactin and dynamin are required for the clathrin-independent endocytosis of gammac cytokine receptor. J. Cell Biol. 168, 155–163 (2005)
Maldonado-Báez, L., Williamson, C. & Donaldson, J. G. Clathrin-independent endocytosis: a cargo-centric view. Exp. Cell Res. 319, 2759–2769 (2013)
Grassart, A., Dujeancourt, A., Lazarow, P. B., Dautry-Varsat, A. & Sauvonnet, N. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep. 9, 356–362 (2008)
Fritsch, R. & Downward, J. SnapShot: Class I PI3K isoform signaling. Cell 154, 940 (2013)
Servant, G. et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040 (2000)
Song, M. S., Salmena, L. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor. Nature Rev. Mol. Cell Biol. 13, 283–296 (2012)
Xie, J., Erneux, C. & Pirson, I. How does SHIP1/2 balance PtdIns(3,4)P2 and does it signal independently of its phosphatase activity? Bioessays 35, 733–743 (2013)
Batty, I. H. et al. The control of phosphatidylinositol 3,4-bisphosphate concentrations by activation of the Src homology 2 domain containing inositol polyphosphate 5-phosphatase 2, SHIP2. Biochem. J. 407, 255–266 (2007)
Gewinner, C. et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 16, 115–125 (2009)
Posor, Y. et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 499, 233–237 (2013)
Yoon, Y., Zhang, X. & Cho, W. Phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) specifically induces membrane penetration and deformation by Bin/amphiphysin/Rvs (BAR) domains. J. Biol. Chem. 287, 34078–34090 (2012)
Krause, M. et al. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev. Cell 7, 571–583 (2004)
Vehlow, A. et al. Endophilin, Lamellipodin, and Mena cooperate to regulate F-actin-dependent EGF-receptor endocytosis. EMBO J. 32, 2722–2734 (2013)
Kononenko, N. L. et al. Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron 82, 981–988 (2014)
Watanabe, S. et al. Ultrafast endocytosis at mouse hippocampal synapses. Nature 504, 242–247 (2013)
McMahon, H. T. & Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature Rev. Mol. Cell Biol. 12, 517–533 (2011)
Boucrot, E. et al. Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell 149, 124–136 (2012)
Römer, W. et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 450, 670–675 (2007)
Ewers, H. et al. GM1 structure determines SV40-induced membrane invagination and infection. Nature Cell Biol. 12, 11–18 (2010)
Renard, H.-F. et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature http://dx.doi.org/10.1038/nature14064 (2014)
Shaner, N. C. et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nature Methods 5, 545–551 (2008)
Usami, Y., Popov, S. & Gottlinger, H. G. Potent rescue of human immunodeficiency virus type 1 late domain mutants by ALIX/AIP1 depends on its CHMP4 binding site. J. Virol. 81, 6614–6622 (2007)
Ford, M. G. et al. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051–1055 (2001)
Marks, B. et al. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410, 231–235 (2001)
Henne, W. M. et al. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science 328, 1281–1284 (2010)
Motley, A., Bright, N. A., Seaman, M. N. & Robinson, M. S. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162, 909–918 (2003)
Subtil, A., Hemar, A. & Dautry-Varsat, A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J. Cell Sci. 107, 3461–3468 (1994)
Pelish, H. E. et al. Secramine inhibits Cdc42-dependent functions in cells and Cdc42 activation in vitro. Nature Chem. Biol. 2, 39–46 (2006)
Macia, E. et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 (2006)
Howes, M. T. et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 190, 675–691 (2010)
Hill, T. A. et al. Inhibition of dynamin mediated endocytosis by the dynoles–synthesis and functional activity of a family of indoles. J. Med. Chem. 52, 3762–3773 (2009)
Joshi, S. et al. The dynamin inhibitors MiTMAB and OcTMAB induce cytokinesis failure and inhibit cell proliferation in human cancer cells. Mol. Cancer Ther. 9, 1995–2006 (2010)
Schlegel, R., Dickson, R. B., Willingham, M. C. & Pastan, I. H. Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of alpha 2-macroglobulin. Proc. Natl Acad. Sci. USA 79, 2291–2295 (1982)
Wang, L. H., Rothberg, K. G. & Anderson, R. G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117 (1993)
Gibson, A. E., Noel, R. J., Herlihy, J. T. & Ward, W. F. Phenylarsine oxide inhibition of endocytosis: effects on asialofetuin internalization. Am. J. Physiol. 257, C182–C184 (1989)
Heuser, J. E. & Anderson, R. G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108, 389–400 (1989)
Larkin, J. M., Brown, M. S., Goldstein, J. L. & Anderson, R. G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell 33, 273–285 (1983)
Yip, S. C. et al. Quantification of PtdIns(3,4,5)P3 dynamics in EGF-stimulated carcinoma cells: a comparison of PH-domain-mediated methods with immunological methods. Biochem. J. 411, 441–448 (2008)
Acknowledgements
We thank S. Y. Peak-Chew for mass spectrometry, M. Edwards and K. McGourty for technical help and P. De Camilli, T. Kirchhausen, G. B. Hammond, P. Robinson, M. Robinson, M. McNiven, B. Nichols, A. Benmerah, M. Krause and Genentech for the gift of reagents, and the members of the McMahon and Boucrot laboratories for comments. The research was funded by the Medical Research Council UK (grant number U105178805) (H.T.M., L.A.-S., G.H., Y.V. and E.B. in part) and a Royal Society grant (research grant number RG120481) (E.B.). A.P.A.F is supported by the Fundação para a Ciência e Tecnologia, L.A.-S. is a EMBO Long Term fellow and is supported by Marie Curie Actions, and E.B. is a Biotechnology and Biological Sciences Research Council (BBSRC) David Phillips Research Fellow.
Author information
Authors and Affiliations
Contributions
H.T.M. and E.B. designed the research and supervised the project. H.T.M. and A.P.A.F. performed pull-down experiments. L.A.-S., A.P.A.F. and S.D. did the signalling experiments. L.A.-S. did the super-resolution imaging. A.P.A.F. did the cell migration experiments. S.D. performed the plasma membrane isolations; Y.V. performed the PC12 cell experiments. G.H. prepared and acquired the electron-microscopy data. L.B. and N.S. provided critical reagents. E.B. performed and analysed all the other experiments. E.B. and H.T.M. wrote the manuscript with input from all the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
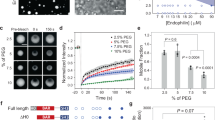
Extended Data Figure 1 Endophilin binds to some GPCRs and is dispensable for clathrin-mediated endocytosis.
Related to Fig. 1. a, Quantification of the binding of indicated cytosolic fragments of adrenergic, dopaminergic, serotoninergic, muscarinic (Muscar.) and histaminergic (Histam.) receptors (see Fig. 1a for an example of one set of immunoblots) to the indicated GST–SH3 domains (or GST as control). The percentage enrichment of receptor tails in pulled-down fractions with respect to their respective levels in the original cell extracts (input) is shown (mean ± s.e.m., n = 3 independent experiments). b, Binding of GST–SH3 domains from BAR and F-BAR containing proteins to β1-AR third intracellular loop (TIL), showing specificity for endophilin-A SH3 domains. c, Pull-down experiments using GST–SH3 domains of endophilin-A2 (or GST as a control) with EGFP-tagged fragments coding for the TILs of the indicated receptors bearing the depicted mutations. Mutations decreasing binding are shown in red; the ones without significant effects are in blue. ALIX PRD domain and its R757E mutant58 were used as positive and negative controls. d, Amino acid sequence around point mutations tested showing potential endophilin-binding motifs. e, Quantification of the binding of indicated mutated cytosolic fragments (see c for an example of one set of immunoblots) to the indicated GST–SH3 domains (or GST as control). The percentage enrichment of receptors tails in pulled-down fractions with respect to their respective levels in the original cell extracts (input) is shown (means ± s.e.m. from three independent experiments; NS, non-significant, *P < 0.05, ***P < 0.001, one-way ANOVA and Dunnett’s test versus respective wild-type). f, Verification of AP2 and Endo1+2+3 depletion by RNAi from three independent control (‘C’) and RNAi (‘R’) experiments. Immunoblot (left) and bar graph (right) representations of the data are shown (means ± s.e.m. from three independent experiments; ***P < 0.001, Student’s t-test versus respective control). g, Transferrin and LDL uptake was inhibited by clathrin and AP2 RNAi but not by control (Ctrl) or endophilin-A triple RNAi (TKD, three different pools). Ligand uptake was measured by flow cytometry (mean ± s.e.m., 10,000 cells per experiment, n = 3 independent experiments; NS, non-significant, ***P < 0.001, one-way ANOVA and Dunnett’s test versus respective control). h, Anti-LDLR antibody uptake assay in control or TKD RPE1 cells. Cells were immunostained for anti-LDLR (post feeding) and endophilin (red). Cells strongly depleted for endophilin (*) showed similar LDLR endocytosis to control cells, showing that LDLR endocytosis is not dependent on endophilin-A proteins (images representative of ten captures). i, Dynamics of clathrin-coated pits in BSC1 cells marked by σ2-EGFP (AP2) and acquired by live-cell spinning-disk confocal microscopy imaging. Left pictures show representative images from the bottom surface of cells; right pictures are kymographs (time projections) representative of five captures from control and Endo1+2+3 RNAi cells. Right: plot of individual lifetimes of clathrin/AP2 pits calculated from live imaging of 4 cells for each condition. Those had significant longer lifetimes in TKD cells, perhaps related to a reduced synaptojanin recruitment to membranes and thus an increase in PtdIns(4,5)P2 levels5,20 (bars, means; n = 211 and 363 from 3 independent experiments, mean ± s.e.m. is written at the bottom, ***P < 0.001, Mann–Whitney U-test). j, Surface staining of β1-AR using the HA–β1-AR–EGFP construct. Control, AP2 RNAi and endophilin TKD cells were directly stained for steady-state accumulation of β1-AR at their cell surface and imaged using a confocal microscope (images are representative of five captures). In endophilin TKD cells β1-AR accumulated in a limited area of the plasma membrane, similar to the area stained with lamellipodin in Extended Data Fig. 2c. This panel complements that in Fig. 1b showing a lack of internalization of β1-AR in TKD cells. k, Immunoblots showing the expression of β1 adrenergic receptor (β1-AR) in BSC1 and RPE1 cells. Decreasing amount of cell extracts were loaded from left to right. l, Left: isolation of a plasma membrane fraction (see Methods) showed accumulation of β1-adrenergic receptors after endophilin RNAi. Erk was used to monitor cytosolic proteins, calnexin endoplasmic reticulum proteins and integrin β1, β1-adrenergic receptor (β1-AR) and EGFR to monitor plasma membrane proteins. Note that the plasma membrane fraction (PM) was enriched in plasma membrane proteins and did not contain cytosolic ERK or ER-localized calnexin. Right: levels of β1-AR were enriched in the plasma membrane fraction from TKD cells versus control cells. m, Bar graph shows the amounts of internalized/total depicted HA–receptor–EGFP in control (grey) or endophilin TKD (red) samples (mean ± s.e.m., n = 3 independent experiments; ***P < 0.001, Student’s t-test versus respective control). n, Surface staining of HA–receptor–EGFP constructs (see Fig. 1b, c). Cells were directly stained with anti-HA antibody at 4 °C and measured using a plate reader to assay the steady-state accumulation of receptors at the cell surface. The cell surface signals (HA) were corrected for expression levels (EGFP) and the values were normalized to the mean of the control cells (mean ± s.e.m., n = 3 independent experiments; ***P < 0.001, Student’s t-test versus respective control). The same receptors that showed a decreased endocytosis (m) show an increased surface accumulation here, an effect that is dependent on ligand activation. Scale bars: 20 μm (h), 10 μm (j) and 5 μm (i).
Extended Data Figure 2 Endophilin and clathrin did not co-localize at the leading edge of cells by standard and super-resolution microscopy.
Related to Fig. 1. a, b, Confocal microscopy images (optical planes from the middle of cells) showing endogenous staining of endophilin (green) in BSC1 cells (low magnification in a and higher magnification in b, left panel) and in a normal diploid RPE1 cell (middle). Images in b were oriented with leading edges (identified by phalloidin staining (actin, red)) pointing to the right. Arrowheads point to endophilin puncta on the leading edge of these cells. Right: there was no anti-endophilin staining in endophilin-A TKD cells, validating the specificity of the antibody. Insets are zooms of the boxed areas. c, Confocal microscopy images showing endogenous staining of lamellipodin, Arp3 or vinculin (all green) and endophilin (red) in BSC1 cells. Insets are zooms of the boxed areas. Endophilin co-localized with lamellipodin and Arp3 at the leading edge but not with vinculin (a marker of focal adhesions). d, Top: same data as in Fig. 1c but showing the individual channels. Confocal microscopy images showing clathrin (EGFP–LCa, green) and endophilin (endophilin A2–RFP, red) in a live BSC1 cell. ‘N’ denotes the nucleus. Bottom: confocal microscopy images showing endogenous staining of α-adaptin (AP2), and endophilin (red) in BSC1. Endogenous staining also shows an enrichment of endophilin at the leading edge. e, Super-resolution stimulated emission depletion (STED) microscopy images of a BSC1 cell immunostained for clathrin and showing coated pits and vesicles labelled at the ventral surface of the cell but not at the leading edges where the staining was diffused. f, Super-resolution structured illumination microscopy (SIM) images of a BSC1 cell immunostained for clathrin (red) and endophilin (green). Note the absence of co-localization between the two markers. g, Confocal microscopy images (bottom surface optical section) showing the localization of AP2 (σ2-EGFP, green) and endophilin (EndoA2–RFP, red) in a live migrating cell. Arrowheads point to endophilin A2 puncta at the leading edge of the cell; these are negative for AP2. Inset is a zoom of the boxed area. h, Confocal microscopy images showing a lack of co-localization of clathrin (EGFP–LCa, green) and the other endophilin-A expressed in BSC1 cells: endophilin A3 (endophilin A3–RFP, red). Arrowheads point to endophilin A3 puncta at the leading edge of live BSC1 cells; these are negative for clathrin puncta. i, Confocal microscopy images (optical section of bottom surface) showing clathrin (EGFP–LCa, green) and endophilin (EndoA2–RFP, red) localization in a live confluent BSC1 cell (thus no leading edge). Neighbouring cells were not transfected. Arrowheads point to endophilin A2 puncta at the bottom surface of the cell; most of these are negative for AP2. Inset is a zoom of the boxed area. j, Representative kymographs of AP2 (σ2–EGFP) and endophilin (EndoA2–RFP) from live-cell imaging acquired at the ventral surface of a confluent cell. The arrowhead points to an endophilin-positive track devoid of AP2. Images and kymographs are representative of at least ten captures, from three independent experiments. Scale bars: 20 μm (a, d), 10 μm (b, c, f, e), 5 μm (e, h) and 1 μm (f insets).
Extended Data Figure 3 Endophilin co-localized with dynamin, cortactin and synaptojanin but not with known clathrin-independent endocytosis markers.
Related to Fig. 1. a, Representative confocal images showing the co-localization of endophilin (endoA2–RFP or anti-endophilin, red) with dynamin (dynamin2–EGFP expressed at low levels, or anti-dynamin for endogenous protein (green)), cortactin (anti-cortactin for endogenous protein) or synaptojanin (synaptojanin 1-170–EGFP expressed at low levels). Insets are zooms of the boxed areas. b, Representative confocal images showing the absence of co-localization between endophilin (anti-endophilin, or endoA2–RFP, red) and caveolin 1 (endogenous), GRAF1–EGFP and flotillin 1–EGFP. The bottom row shows the background co-localization of endophilin with a soluble protein (EGFP). Arrowheads point to endophilin puncta at the leading edge of cells; these were negative for the markers tested. Insets are magnifications of the boxed areas. c, Co-localization of endogenous endophilin (red) with lamellipodin, but not caveolin-1 (green). Images were oriented with the leading edges to the right. Arrowheads point to co-localization between markers. Intensity profiles were acquired along the indicated lines. d, Receptor uptake assay (HA–receptor–EGFP internalized corrected for their total levels) in cells pre-treated with the indicated RNAi (mean ± s.e.m., n = 3 independent experiments). e, Confocal images of cells treated with caveolin-1 (Cav1) or flotillin 1 and 2 (Flot1+2) or control siRNA and immunostained for endophilin (green) and actin (red). Cells were counter-stained (blue) for caveolin 1 (Cav1) or flotillin-1 (Flot1) to ascertain for the depletion of the targeted proteins in the cells imaged. Images are representative of at least ten captures, from three independent experiments. Scale bars: 10 μm (a) and 5 μm (b, c and e).
Extended Data Figure 4 Formation of endophilin-positive tubules and vesicles upon β1-adrenergic receptor activation.
Related to Fig. 1. a, Images from a time-lapse acquired by spinning-disk confocal microscopy of a BSC1 cell expressing low levels of endophilin-A2–RFP, after addition of 10 μM of denopamine (t = 0). Arrowheads point to a tubulo-vesicular carrier formed upon stimulation and moving from the cell edge (right) to the cell centre (left). See also Supplementary Video 3. b, The budding rate of endophilin-positive assemblies (EPAs), measured as number of EPAs μm−2 s−1, after stimulation with denopamine (added at t = 0). Data were acquired from data sets similar to Supplementary Video 3 and normalized to the maximum budding rate (mean ± s.e.m., n = 3 independent experiments). c, Budding of endophilin–mRFP-positive structures determined by live-cell imaging after addition of various β1-AR (isoproterenol, dobutamine and denopamine) or β2-AR (isoproterenol, terbutaline) agonists concentrations. Data are expressed as the percentage of maximum budding. See also Supplementary Video 3. Terbutaline, the specific β2-AR agonist, does not stimulate EPA production. Inhibition of clathrin-mediated endocytosis by AP180 C terminus expression does not affect EPA formation stimulated by the β1-AR agonist denopamine (mean ± s.e.m., n = 3 independent experiments). d, Representative confocal images (optical planes located at the middle of cells) showing the co-localization of endophilin A2–RFP (red) with endogenous endophilin (green) in a BSC1 cell stimulated with denopamine (10 μM for 4 min), validating the targeting of exogenously expressed endophilin in our experiments. e, Representative confocal images (optical section located at the middle of cells) of confluent cells stimulated for 4 min with 10 μM denopamine and stained for endogenous endophilin (green) and α-adaptin (AP2, red). f, Representative confocal images (optical section located at the middle of cells) of sparse (f) or confluent (e) cells stimulated for 4 min with the indicated adrenergic agonists and stained for endogenous endophilin (green) and actin (phalloidin, red). g, Quantification of EPAs in BSC1 and RPE1 cells upon stimulation with adrenergic receptor agonists in control, AP2 RNAi or AP180 dominant-negative-expressing cells or in cells pre-treated (5 min) with beta-blockers before stimulation (mean ± s.e.m., n = 3 independent experiments). h, Quantification of the number of endogenous EPAs per 400 μm2 in BSC1, RPE1 cells and in primary fibroblasts upon stimulation with 10 μM denopamine for the indicated amounts of time (mean ± s.e.m., n = 3 independent experiments). i, Immuno-electron microscopy of BSC1 cells fixed 4 min after addition of 10 μM denopamine (deno) to the medium or in absence of stimulation (control). Anti-endophilin antibodies were detected with a gold-conjugated secondary antibody (red balls). The two pictures on the left show clathrin-coated pits (top) and a potential vesicle (bottom). The other images are membrane structures at the plasma membrane and in the cytoplasm positive for the anti-endophilin antibody (endogenous endophilin). j, Representative confocal images (optical section located at the middle of cells) of cells depleted for clathrin (left, same image as in Fig. 1e) or AP2 and stimulated with denopamine (10 μM) for 4 min before fixation. Endogenous endophilin (green) clathrin or AP2 (blue) and actin (phalloidin, red) were immunostained. k, Confocal microscopy images of a stimulated (10 μM denopamine for 4 min) RPE1 cell grown on a poly-lysine-coated coverslip, incubated in soluble lysine-fixable Alexa647 dye, fixed and immunostained for endophilin (green). On the confocal plane taken at the bottom of the cell (dye in focus), no endophilin spots can be seen (out of focus). On a confocal plane at the middle of the cell (dye outlines the cell cross-section) EPAs are in focus, indicating that they are in the cytoplasm and not at the cell surface. l, Example of a BSC1 cell treated and immunostained as in m and imaged at the middle of the cell and showing several endophilin-positive tubules and diffraction-limited puncta. m, Confocal microscopy images of a stimulated (10 μM denopamine for 4 min) BSC1 cell fixed and immunostained for α-adaptin (AP2, red) and endophilin (green). On the confocal plane taken at the bottom of the cell AP2 spots (known to be located at the plasma membrane) are in focus but EPAs are out of focus. On a confocal plane at the middle of the cell AP2 spots are out of focus but EPAs are in focus, indicating that they are inside the cytoplasm and not at the plasma membrane. n, Confocal microscopy images of EPAs labelled by endophilin. Arrowheads show diffraction-limited punctates, tubules and ‘doughnut-like’ vesicles structures. o, Quantification of the occurrence in stimulated BSC1, RPE1 and human primary dermal fibroblasts of the three different endophilin-positive structures: diffraction-limited punctates (grey), tubules (green) and ‘doughnut-like’ vesicles (mean ± s.e.m., n = 3 independent experiments). Images are representative of at least ten captures, from three independent experiments. Scale bars: 5 μm apart from i, which is 250 nm.
Extended Data Figure 5 β1 and β2 arrestins were not essential in β1-adrenergic receptor agonist stimulation of endophilin vesicle formation.
Related to Fig. 1. a, Representative confocal images (optical planes located at the middle of cells) of cells overexpressing the indicated EGFP-tagged receptors TIL (green), stimulated with 10 μM denopamine for 4 min and immunostained for endophilin (red) (images are representative of ten captures from three independent experiments). b, Quantification of the number of endogenous EPAs in cells overexpressing the indicated EGFP-tagged receptors TIL and stimulated with 10 μM denopamine for 4 min (mean ± s.e.m., n = 3 independent experiments; NS, non-significant, *P < 0.05, ***P < 0.001, one-way ANOVA and Dunnett’s test versus GFP). c, Confocal microscopy images of control and endophilin TKD cells, resting or stimulated (10 μM denopamine for 4 min) and immunostained for phosphorylated CREB (pCREB, green), endophilin (red) and DNA (DRAQ5, blue) (images are representative of ten captures from three independent experiments). Asterisks denote cells with strongly reduced endophilin levels. d, Plate reader quantification of BSC1 or RPE1 cells treated as in a (mean ± s.e.m., n = 3 independent experiments; NS, non-significant, Student’s t-test versus respective control). e, Confocal microscopy images of control and stimulated (10 μM denopamine for 4 min) cells immunostained for β-arrestin (green) and endophilin (red). EPAs (arrowheads) did not contain β-arrestin (images representatives of ten captures from three independent experiments). f, g, Confocal microscopy images of cells overexpressing β-arrestin-1 or 2-EGFP (f, green) or β-arrestin 1+2 KD cells (g), stimulated with denopamine (10 μM for 4 min), fixed and immunostained for endophilin (red) and β-arrestin (g, green) (images representatives of at least ten captures). h, Plate reader quantification of BSC1 or RPE1 cells treated as in e–g (mean ± s.e.m., n = 3 independent experiments; NS, non-significant, one-way ANOVA and Dunnett’s test versus respective controls). Scale bars: 40 μm (c) and 5 μm (a, e–g).
Extended Data Figure 6 Several growth factors stimulated the formation of endophilin-positive assemblies.
Related to Fig. 2. a, Confocal images (optical planes from the middle of cells) of BSC1 cells treated for 4 min with the indicated growth factors (all at 10 ng ml−1) and immunostained for endogenous endophilin-A (green). Cells were oriented with their leading edges (identified with phalloidin staining (actin, red)) pointing to the right (images are representative of ten captures from three independent experiments). Arrowheads show internal vesicles positive for endogenous endophilin in the VEGF, PDGF and IGF-1-treated but not TGF-β-treated cells (see also quantification in Fig. 2c). b, EPA production in a primary fibroblast from an adult donor treated for 4 min with 50 ng ml−1 EGF and immunostained for endogenous endophilin-A (green). The image is representative of eight captures. c, Quantification of the number of endogenous EPAs per 400 μm2 in BSC1, RPE1 cells or primary fibroblasts upon stimulation with 50 ng ml−1 EGF for the indicated amounts of time (mean ± s.e.m., n = 3 independent experiments). d, Confocal section of a BSC1 cell fixed 4 min after addition of 50 ng ml−1 Alexa555-labelled EGF, HGF, FGF, VEGF and IGF-1 to the medium. Endogenous endophilin (green) was detected as in a. Arrowheads point to internal vesicles positive for endogenous endophilin and containing internalized growth factors (images are representatives of at least eight captures). e, Plate reader quantification of BSC1 cell incubated with a monoclonal antibody anti-EGFR (13A9, green), that does not interfere with EGF binding, and 50 ng ml−1 of the indicated ligands. There is no cross-talk between EGFR and activation of other growth factor receptors or activation of β1 adrenergic receptors. Thus, endophilin-positive structures only have EGFR when stimulated by EGF (see Fig. 2d, e), and likewise on β1 adrenergic receptor activation there is no EGFR in the endophilin-positive structures. This reinforces the theme that one needs specific receptor activation to transduce a signal across the membrane to activate EPA formation (mean ± s.e.m., n = 3 independent experiments). f, Quantification of the number of EPAs in cells depleted for CIN85 or Cbl or overexpressing β1-AR TIL, Cbl-PRD or CIN85-PRD, -CC-PRD, SH3(3) and stimulated with EGF (blue), HGF (purple) or denopamine (fuchsia) (mean ± s.e.m., n = 3 independent experiments). g, Confocal images of control, AP2, clathrin or endophilin TKD (pools 1 and 3) treated cells, fixed and immunostained for EGFR (green) and endophilin (red) (images are representative of at least ten captures). Asterisks denote cells with strongly reduced endophilin levels. h, Plate reader quantification of BSC1 and RPE1 treated as in g (mean ± s.e.m., n = 3 independent experiments). i, Flow cytometry profiles of total EGFR levels in control (black) or TKD (red) cells. n = 50,000 cells for each conditions. j, Plate reader quantification of control or endophilin TKD BSC1 and RPE1 treated with 50 ng ml−1 EGF for the indicated time (mean ± s.e.m., n = 3 independent experiments). k, Confocal images of a BSC1 cell stimulated for 5 min with 250 ng ml−1 EGF, fixed and immunostained for EGFR (green) and endophilin (red) (images are representative of eight captures). Note that EPAs (arrowheads) are distinct from the large vacuole-like structures which are called macropinosomes, seen best in the middle confocal plane. Scale bars: 40 μm (g), 10 μm (k) and 5 μm (a–c).
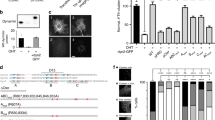
Extended Data Figure 7 MAPK signalling and neurite outgrowth in endophilin TKD cells.
Related to Fig. 2. a, Signals from PathScan antibody arrays reporting various intracellular signalling pathways. Intensities were normalized to the mean of the controls. Of all the signalling pathways tested endophilin TKD leads to a higher level of ERK phosphorylation (a component of the MAP kinase signalling cascade) in resting cells (mean, n = 2 independent experiments). b, Signals from PathScan antibody arrays reporting various steps within the EGFR signalling cascade. Intensities were normalized to the mean of the controls. The inset shows the level of endophilin knockdown in TKD cells. Endophilin TKD does not prevent phosphorylation of the EGFR at Y998 and thus does not account for defective endocytosis. Endophilin TKD does not prevent MEK phosphorylation on the RAF phosphorylation site (S217/S221), important for MEK activation (mean, n = 2 independent experiments). c, Immunoblots from control (C), endophilin TKD (E) or AP2 RNAi (A) cells stimulated with 5 (left) or 100 ng ml−1 (right) EGF for the indicated times. Quantification of the signals from three independent experiments, normalized to the mean of the controls at t = 0 are shown below. As in a the resting pERK levels are increased in endophilin RNAi cells, probably because of an accumulation of receptors on the cell surface. At low concentrations of EGF (5 ng ml−1) AP2 RNAi largely prevents MAP kinase signalling while endophilin RNAi does not (but rather an enhanced level of signalling). At higher concentrations of EGF (100 ng ml−1) there is much stronger signalling at an early time point in endophilin RNAi, pointing again to the importance of this pathway in reducing basal signalling (mean ± s.e.m., n = 3 independent experiments; ***P < 0.001, one-way ANOVA and Dunnett’s test versus control t = 0). d, Confocal microscopy images of resting or stimulated (50 ng ml−1 EGF for 10 min) cells pre-treated with endophilin TKD (3 days) or the indicated inhibitors for 30 min before stimulation and immunostained for phosphorylated ERK1/2 (pERK, red), endophilin (green) and DNA (DRAQ5, blue) (images are representatives of at least ten captures). Asterisks denote cells with strongly reduced endophilin levels. e, Flow cytometry analysis of phosphorylated Erk1/2 levels in resting or stimulated (50 ng ml−1 EGF for 10 min) control (black) and endophilin TKD (red) cells. n = 50,000 cells for each conditions. f, Plate reader quantification of BSC1 or RPE1 cells treated as in d. Thus by immunoblotting and by immunostaining there is an increase in pERK in resting conditions in endophilin RNAi cells. Note the stronger increase in basal and stimulated pERK levels in endophilin TKD cells (mean ± s.e.m., n = 3 independent experiments). g, Plate reader quantification of control and endophilin TKD BSC1 or RPE1 cells resting or stimulated (50 ng ml−1 EGF for 30 min) and immunostained for phosphorylated Elk1, Jun or CREB (pElk1, pJun or pCREB) (mean ± s.e.m., n = 3 independent experiments). h, Left: confocal images (optical planes from the middle of cells) of BSC1 cells pre-treated with MEKi 1 or MEKi 2 for 30 min and stimulated with EGF (50 ng ml−1, 4 min) and immunostained for endogenous endophilin-A (green) (images are representatives of six captures). Endophilin-positive puncta formation in response to growth factors is not dependent on MEK activation. Right: quantification of the number of endogenous EPAs per 400 μm2 in BSC1 or RPE1 cells pre-treated for 30 min with the indicated inhibitors and stimulated with EGF (50 ng ml−1, 4 min) (mean ± s.e.m., n = 3 independent experiments). i, NGF stimulates endophilin-positive puncta formation is RPE1 cells. Confocal images (optical planes from the middle of cells) of an RPE1 cell treated for 4 min with NGF (10 ng ml−1 for 4 min), fixed and immunostained for endogenous endophilin-A (green) (images are representatives of six captures). Cells were oriented with the leading edges (identified with phalloidin staining (actin, red)) pointing to the right. Arrowheads show internal vesicles positive for endogenous endophilin. j, Confocal images of cells treated with endophilin (endo TKD) or control siRNA and incubated with anti-NGFR antibodies (green) and 10 ng ml−1 NGF for 10 min at 37 °C, surface stripped on ice, fixed and immunostained for endophilin (red) and actin (blue) (images are representatives of eight captures). k, Neurite extension assays. PC12 cells pre-treated with control or endophilin TKD were stimulated or not with 100 ng ml−1 NGF for up to 6 days (images are representatives of at least 12 images). Quantifications show the percentage of cells with neurites (top left), average number of neurites per cell (top right), average neurite length (bottom left) and average neurite length per cell having neurites (bottom right) (mean ± s.e.m., n = 3 independent experiments). Scale bars: 40 μm (d), 20 μm (k) and 5 μm (h–j).
Extended Data Figure 8 IL-2R uptake in endophilin TKD human T cells and chemical and genetic perturbations of FEME.
Related to Figs 3 and 4. a, Confocal microscopy images (focal plane at the middle of cells) of human T cells immunostained for endogenous endophilin A (green) (images are representatives of at least ten images). Cells were either fixed directly (‘resting’) or 3 and 10 min after addition of IL-2 (500 pM). Scale bars, 5 μm (mean ± s.e.m., n = 3 independent experiments; ***P < 0.001, Student’s t-test versus respective controls). b, Internalization rates of IL-2R and TfR in control, AP2 or endophilin TKD human T cells, measured by a plate reader (mean ± s.e.m., n = 3 independent experiments; ***P < 0.001, Student’s t-test versus respective control time points). Inset: immunoblots of cell extracts used validated endophilin TKD. c, Super-resolution structured illumination microscopy (SIM) images of BSC1 cells overexpressing RFP-dynamin1-T65A mutant and immunostained for endophilin (green) and clathrin (blue) (images are representatives of at least five captures). Note that there was no leading edge in the presence of this dynamin mutant, and endophilin-positive assemblies were distributed across the plasma membrane. Dynamin1-T65A formed short tubules from the plasma membrane and at the tips of some of these tubules (arrowheads) in the boxed area (edges of cells) there were endophilin but not clathrin signals. d, Co-immunoprecipitation of ΔH0-BAR–EGFP with endogenous endophilin (red box, left) of with Myc-tagged overexpressed endophilin (red box, right). e, Flow cytometry profiles of internalized transferrin (Alexa546-labelled) in cells overexpressing increasing amounts of EGFP–AP180-DN (red) or ΔH0-BAR–EGFP (black). n = 2,000 cells for each condition. f, Confocal images of cells overexpressing the indicated mutants (blue) for 24 h or treated with the indicated inhibitors for 5 min before stimulation with 10 μM denopamine (4 min), fixed and immunostained for endogenous endophilin-A (green) and actin (phalloidin, red) (images are representative of at least six captures). Arrowheads point to EPAs. g, Live-cell confocal images before and after (+30 s) the addition of indicated inhibitors to cells expressing endophilin A2–RFP (green) and clathrin (EGFP–LCa, red) or dynamin (dynamin 2–EGFP, red) (images are representatives of at least three captures from independent experiments). Arrowheads point to endophilin spots. Scale bars: 10 μm (a, c), 5 μm (f, g) and 1 μm (c, insets).
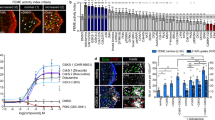
Extended Data Figure 9 The recruitment of endophilin at leading edges of cells requires PtdIns(3,4)P2.
Related to Fig. 5. a, Receptor uptake assay (HA–receptor–EGFP internalized, corrected for total levels) in cells treated with the depicted inhibitors (mean ± s.e.m., n = 3 independent experiments). b, Wound-closure assay. BSC1 and RPE1 cell monolayers were wounded and cell migration into the wound sites were assessed after 16 h. The inhibitors were added just after wounding (mean ± s.e.m., n = 3 independent experiments). c, Confocal images of cells treated with the indicated inhibitors or RNAi, fixed and immunostained for endogenous endophilin-A (green) and actin (phalloidin, red) (images are representative of at least six captures from three independent experiments). Arrowheads point to endophilin foci at the leading edges of cells. d, Confocal images of cells overexpressing the indicated constructs fixed and immunostained for endogenous endophilin-A (red) and actin (phalloidin, blue) (images are representative of at least six captures from three independent experiments). e, Quantifications of PtdIns(4,5)P2 (mocha), PtdIns(3,4)P2 (red) or PtdIns(3,4,5)P3 (asparagus) levels from cells treated with the indicated siRNA and PI(3)K inhibitor, immunostained for the lipids and measured using a plate reader (mean ± s.e.m., n = 3 independent experiments; NS, non-significant; *P < 0.05; ***P < 0.001, one-way ANOVA and Dunnett’s test versus respective control levels). f, Confocal images of cells treated with INP4A and INP4B or control siRNA, fixed and immunostained for endogenous endophilin-A (green) and LAMP-1 (red) (images are representative of at least six captures from three independent experiments). Intensity profiles were acquired along the indicated lines. Scale bars: 20 μm (d), 10 μm (f) and 5 μm (c).
Extended Data Figure 10 Lamellipodin recruits endophilin at the leading edge.
Related to Fig. 5. a, Scheme depicting the endophilin constructs used. b, c, Confocal images of cells depleted for endogenous endophilin (TKD) or not (control siRNA) and expressing the indicated constructs, fixed and immunostained for endogenous endophilin to ascertain the depletion in the cells imaged (images are representatives of at least six captures from three independent experiments). d, Quantification of construct levels at the leading edges of cells depleted of endogenous endophilin (TKD) and expressing the indicated endophilin constructs (mean ± s.e.m., n = 3 independent experiments; NS, non-significant; ***P < 0.001 one-way ANOVA and Dunnett’s test versus respective full-length levels). e, Pull-down experiments using GST–SH3 domains of endophilin-A1, 2 or 3 (or GST as a control) with EGFP–lamellipodin. 10% of cell extract was used as ‘input’. f, Confocal image of cell depleted for lamellipodin (lpd) and treated with 200 ng ml−1 EGF for 5 min to stimulate PI(3)K and induce ruffles, fixed and immunostained for endophilin (green), actin (red) and lamellipodin (blue, to ascertain the depletion) (images are representatives of at least six captures from three independent experiments). g, Confocal image of a cell overexpressing EGFP–lamellipodin next to a non-transfected cell, fixed and immunostained for endophilin (red) and actin (blue) (images representatives of at least six captures from three independent experiments). h, Confocal images of cells treated with the indicated RNAi and inhibitors, fixed and immunostained for lamellipodin (green), endophilin (red) and actin (blue) (images representatives of at least six captures from three independent experiments). i, Quantification of levels of endogenous lamellipodin at the leading edges of cells treated with the indicated small inhibitors (blue), RNAi (purple), RNAi plus class I PI(3)K inhibitor (‘PI3Ki’, GDC-0941, 50 nM for 5 min; red) (mean ± s.e.m., n = 3 independent experiments; NS, non-significant; *P < 0.05, **P < 0.01, ***P < 0.001 one-way ANOVA and Dunnett’s test versus control). j, Model: endophilin-dependent (FEME) endocytosis from the leading edge. Endophilin-coated vesicles/tubules associate with receptors (after ligand binding) or receptor-adaptors (like CIN85/Cbl) via their SH3 domains, and promote membrane curvature with their N-terminal N-BAR domains. Vesicle scission is promoted by dynamin, which is also recruited by endophilin. Scale bars: 20 μm (b, g), 10 μm (f) and 5 μm (c, h).
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion and Supplementary References. (PDF 306 kb)
Spinning-disk confocal microscopy of a BSC1 cell stably expressing σ2-EGFP (AP2, green) and transiently expressing low levels of endophilin A2-RFP (red) and imaged at 0.5 Hz.
The cell was imaged at 37 °C in normal imaging medium (5 % serum). Note the numerous endophilin puncta devoid of AP2 at the leading edge of the cell. The video is playing at 10 frames/sec. (MOV 6447 kb)
Spinning-disk confocal microscopy of a confluent BSC1 cell transiently expressing low levels of EGFP-LCa (clathrin, green) and endophilin A2-RFP (red) and imaged at 2 Hz.
The cell was imaged at 37 °C in normal imaging medium (5 % serum). Note the numerous endophilin puncta devoid of clathrin. The video is playing at 10 frames/sec. (MOV 6170 kb)
Spinning-disk confocal microscopy (focal plane ~1 μm above the bottom surface) of a BSC1 cell transiently expressing low levels of endophilin A2-RFP (red) and imaged at 0.5 Hz.
The cell was imaged at 37 °C in normal imaging medium (5 % serum). Additional 10 μM isoproterenol was added at the time frame 5. Note the numerous endophilin-coated tubules and vesicles budding from the periphery of the cell and accumulating toward the perinuclear area. The video is playing at 10 frames/sec. (MP4 29175 kb)
Spinning-disk confocal microscopy (focal plane ~1 μm above the bottom surface) of a BSC1 cell transiently expressing low levels of endophilin A2-RFP (red) and imaged at 0.5 Hz.
The cell was imaged at 37 °C in serum-free imaging medium (changed right before imaging). Additional 2 ng/mL was added at the time frame 0. Note the numerous endophilin-coated tubules and vesicles budding from the periphery of the cell and moving toward the perinuclear area. The video is playing at 10 frames/sec. (MP4 16738 kb)
Spinning-disk confocal microscopy (focal plane ~1 μm above the bottom surface) of a BSC1 cell transiently expressing endophilin A2-RFP (red) and Cdc42-T17N dominant negative mutant and imaged at 0.5 Hz.
The cell was imaged at 37 °C in normal imaging medium (5 % serum). Note the recruitment of endophilin all around the edge of the cell and the numerous endophilin-coated tubules and vesicles budding from the periphery of the cell even though the cell was not stimulated with additional growth factor or β1-AR agonist. The video is playing at 10 frames/sec. (MOV 13892 kb)
Rights and permissions
About this article
Cite this article
Boucrot, E., Ferreira, A., Almeida-Souza, L. et al. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 517, 460–465 (2015). https://doi.org/10.1038/nature14067
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14067
This article is cited by
-
Endophilin A2 controls touch and mechanical allodynia via kinesin-mediated Piezo2 trafficking
Military Medical Research (2024)
-
Adhesion energy controls lipid binding-mediated endocytosis
Nature Communications (2024)
-
Membrane transformations of fusion and budding
Nature Communications (2024)
-
Endocytosis in cancer and cancer therapy
Nature Reviews Cancer (2023)
-
Gasdermin D-mediated keratinocyte pyroptosis as a key step in psoriasis pathogenesis
Cell Death & Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.