Abstract
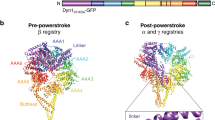
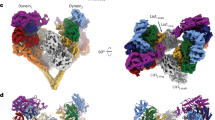
Members of the dynein family, consisting of cytoplasmic and axonemal isoforms, are motors that move towards the minus ends of microtubules. Cytoplasmic dynein-1 (dynein-1) plays roles in mitosis and cellular cargo transport1, and is implicated in viral infections2 and neurodegenerative diseases3. Cytoplasmic dynein-2 (dynein-2) performs intraflagellar transport4 and is associated with human skeletal ciliopathies5. Dyneins share a conserved motor domain that couples cycles of ATP hydrolysis with conformational changes to produce movement6,7,8,9. Here we present the crystal structure of the human cytoplasmic dynein-2 motor bound to the ATP-hydrolysis transition state analogue ADP.vanadate10. The structure reveals a closure of the motor’s ring of six AAA+ domains (ATPases associated with various cellular activites: AAA1–AAA6). This induces a steric clash with the linker, the key element for the generation of movement, driving it into a conformation that is primed to produce force. Ring closure also changes the interface between the stalk and buttress coiled-coil extensions of the motor domain. This drives helix sliding in the stalk which causes the microtubule binding domain at its tip to release from the microtubule. Our structure answers the key questions of how ATP hydrolysis leads to linker remodelling and microtubule affinity regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Roberts, A. J., Kon, T., Knight, P. J., Sutoh, K. & Burgess, S. A. Functions and mechanics of dynein motor proteins. Nature Rev. Mol. Cell Biol. 14, 713–726 (2013)
Dodding, M. P. & Way, M. Coupling viruses to dynein and kinesin-1. EMBO J. 30, 3527–3539 (2011)
Schiavo, G., Greensmith, L., Hafezparast, M. & Fisher, E. M. Cytoplasmic dynein heavy chain: the servant of many masters. Trends Neurosci. 36, 641–651 (2013)
Ishikawa, H. & Marshall, W. F. Ciliogenesis: building the cell’s antenna. Nature Rev. Mol. Cell Biol. 12, 222–234 (2011)
Schmidts, M. et al. Exome sequencing identifies DYNC2H1 mutations as a common cause of asphyxiating thoracic dystrophy (Jeune syndrome) without major polydactyly, renal or retinal involvement. J. Med. Genet. 50, 309–323 (2013)
Burgess, S. A., Walker, M. L., Sakakibara, H., Knight, P. J. & Oiwa, K. Dynein structure and power stroke. Nature 421, 715–718 (2003)
Kon, T., Mogami, T., Ohkura, R., Nishiura, M. & Sutoh, K. ATP hydrolysis cycle-dependent tail motions in cytoplasmic dynein. Nature Struct. Mol. Biol. 12, 513–519 (2005)
Roberts, A. J. et al. ATP-driven remodeling of the linker domain in the dynein motor. Structure 20, 1670–1680 (2012)
Roberts, A. J. et al. AAA+ ring and linker swing mechanism in the dynein motor. Cell 136, 485–495 (2009)
Davies, D. R. & Hol, W. G. The power of vanadate in crystallographic investigations of phosphoryl transfer enzymes. FEBS Lett. 577, 315–321 (2004)
Gibbons, I. R., Gibbons, B. H., Mocz, G. & Asai, D. J. Multiple nucleotide-binding sites in the sequence of dynein β heavy chain. Nature 352, 640–643 (1991)
Kon, T., Nishiura, M., Ohkura, R., Toyoshima, Y. Y. & Sutoh, K. Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry 43, 11266–11274 (2004)
Schmidt, H., Gleave, E. S. & Carter, A. P. Insights into dynein motor domain function from a 3.3-Å crystal structure. Nature Struct. Mol. Biol. 19, 492–497 (2012)
Carter, A. P. Crystal clear insights into how the dynein motor moves. J. Cell Sci. 126, 705–713 (2013)
Imamula, K., Kon, T., Ohkura, R. & Sutoh, K. The coordination of cyclic microtubule association/dissociation and tail swing of cytoplasmic dynein. Proc. Natl Acad. Sci. USA 104, 16134–16139 (2007)
Carter, A. P., Cho, C., Jin, L. & Vale, R. D. Crystal structure of the dynein motor domain. Science 331, 1159–1165 (2011)
Lin, J., Okada, K., Raytchev, M., Smith, M. C. & Nicastro, D. Structural mechanism of the dynein power stroke. Nature Cell Biol. 16, 479–485 (2014)
Kon, T. et al. The 2.8Å crystal structure of the dynein motor domain. Nature 484, 345–350 (2012)
Wendler, P., Ciniawsky, S., Kock, M. & Kube, S. Structure and function of the AAA+ nucleotide binding pocket. Biochim. Biophys. Acta 1823, 2–14 (2012)
Gleave, E. S., Schmidt, H. & Carter, A. P. A structural analysis of the AAA+ domains in Saccharomyces cerevisiae cytoplasmic dynein. J. Struct. Biol. 186, 367–375 (2014)
Vale, R. D., Soll, D. R. & Gibbons, I. R. One-dimensional diffusion of microtubules bound to flagellar dynein. Cell 59, 915–925 (1989)
Gibbons, I. R. et al. The affinity of the dynein microtubule-binding domain is modulated by the conformation of its coiled-coil stalk. J. Biol. Chem. 280, 23960–23965 (2005)
Kon, T. et al. Helix sliding in the stalk coiled coil of dynein couples ATPase and microtubule binding. Nature Struct. Mol. Biol. 16, 325–333 (2009)
Carter, A. P. et al. Structure and functional role of dynein’s microtubule-binding domain. Science 322, 1691–1695 (2008)
Nishikawa, Y. et al. Structure of the entire stalk region of the dynein motor domain. J. Mol. Biol. 426, 3232–3245 (2014)
Redwine, W. B. et al. Structural basis for microtubule binding and release by dynein. Science 337, 1532–1536 (2012)
Liu, Y. et al. Bicaudal-D uses a parallel, homodimeric coiled coil with heterotypic registry to coordinate recruitment of cargos to dynein. Genes Dev. 27, 1233–1246 (2013)
Erzberger, J. P. & Berger, J. M. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 35, 93–114 (2006)
Battye, T. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D 67, 271–281 (2011)
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D 62, 72–82 (2006)
Strong, M. et al. Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 103, 8060–8065 (2006)
Vonrhein, C., Blanc, E., Roversi, P. & Bricogne, G. Automated structure solution with autoSHARP. Methods Mol. Biol. 364, 215–230 (2007)
Kelley, L. A. & Sternberg, M. J. Protein structure prediction on the Web: a case study using the Phyre server. Nature Protoc. 4, 363–371 (2009)
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D 67, 355–367 (2011)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Brunger, A. T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Wallace, A. C., Laskowski, R. A. & Thornton, J. M. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 8, 127–134 (1995)
Waterhouse, A. M., Procter, J. B., Martin, D. M., Clamp, M. & Barton, G. J. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009)
Huecas, S. & Andreu, J. M. Energetics of the cooperative assembly of cell division protein FtsZ and the nucleotide hydrolysis switch. J. Biol. Chem. 278, 46146–46154 (2003)
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007)
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012)
Reck-Peterson, S. L. et al. Single-molecule analysis of dynein processivity and stepping behavior. Cell 126, 335–348 (2006)
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675 (2012)
Acknowledgements
This work was funded by the Medical Research Council, UK (MC_UP_A025_1011), a Wellcome Trust New Investigator Award (WT100387) and an EMBO Young Investigator Award. We thank M. Yu for in-house support with X-ray data collection and Diamond Light Source for access to beamline I02 (MX8547-70). We thank G. Dornan and M. Barczyk for assistance with insect cell culture. We also thank S. Bullock and D. Barford for their advice and comments on the manuscript.
Author information
Authors and Affiliations
Contributions
R.Z. and H.S. screened many dynein species for expression and crystallization. R.Z. expressed human dynein-2 in insect cells, obtained crystals in the presence of vanadate and collected data. H.S. phased the structure and built an initial model. A.P.C. built and refined the structure. R.Z. and H.S. made mutants and performed biochemical assays. L.U. performed negative-stain electron microscopy. H.S., R.Z. and A.P.C. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Examples of the electron density quality in dynein-2:ADP.Vi.
2Fo − Fc electron density in different parts of dynein-2:ADP.Vi. Amino-acid side-chains are clearly resolved in the linker (a), AAA1 (b), AAA4 (c) and AAA6 (d). Only the main-chain could be traced in the stalk (e) and the buttress (f). The electron density in a–d was map-sharpened. The contour level is 1σ, except for e which was contoured at 0.75σ.
Extended Data Figure 2 Structural similarity between individual subdomains of dynein-1 and dynein-2.
Alignment of individual subdomains from dynein-2:ADP.Vi and dynein-1:ADP (PDB accession number 3VKG). a, Alignment of AAA+ large (AAA1L-AAA6L) subdomains and the linker subdomains (Link1–2, Link3–4). b, Alignment of individual AAA+ small subdomains (AAA1S–AAA6S) and the C-terminal domain. Dynein-2 subdomains are coloured according to the scheme used in the main text, and shown in the inset cartoons. Dynein-1 subdomains are shown in grey. Calculated root mean squared deviation (r.m.s.d.) values are shown above each alignment and demonstrate that the subdomains of dynein-2 are structurally highly similar to dynein-1. The AAA+ ring subdomains with the largest r.m.s.d. differences are AAA1L and AAA1S. These subdomains are the most strongly conserved part of the dynein structure and the differences are probably due to the ADP.Vi binding. The distortion of AAA1L, by its interaction with the AAA2L inserts, is described in the main text.
Extended Data Figure 3 Closed interfaces between AAA+ domains of the AAA+ ring in dynein-2:ADP.Vi.
a, Gaps in the AAA+ rings of different dynein motor domain crystal structures. In dynein-1:APO (PDB accession number 4AKI) and dynein-1:ADP (PDB accession number 3VKG) there are gaps between AAA1L/AAA2L and AAA5L/AAA6L or AAA4L/AAA5L. In dynein-2:ADP.Vi a smaller gap exists between AAA5L/AAA6L. Gaps are indicated by black arrows. b, Calculated buried surface areas indicate that the interfaces between AAA1/AAA2, AAA2/AAA3, AAA3/AAA4, AAA4/AAA5 and AAA6/AAA1 are tightly closed in dynein-2:ADP.Vi (buried surface areas 1,059–1,706 Å2). The AAA5/AAA6 interface is more open (buried surface area 837 Å2). Nucleotides are shown in stick representation. AAAL, AAA+ large subdomain; AAAS, AAA+ small subdomain.
Extended Data Figure 4 The four nucleotide binding sites of dynein-2:ADP.Vi.
a–c, The AAA1 site contains electron density consistent with an Mg.ADP.Vi molecule. All catalytic amino-acid residues have the correct conformation to support catalysis. d, Photo cleavage11 of washed dynein-2 crystals upon exposure to ultraviolet light (+UV) produces two bands of 300 and 90 kDa (arrowheads). This suggests crystals contain an ADP.Vi group in AAA1. e, f, The AAA2 site contains density consistent with a Mg.ATP molecule. g, h, The AAA3 and i, j, AAA4 sites contain electron density that is best modelled as ADP. In contrast to AAA1, AAA2–AAA4 have lost the catalytic residues necessary for ATP hydrolysis (the Walker B glutamate, the arginine finger, sensor-I and sensor-II motifs). The Fo − Fc electron density (a, e, g, i) is contoured at 3σ. The 2Fo − Fc electron density (c) is contoured at 1σ. W-A, Walker A motif; W-B, Walker B motif; S-I, sensor-I; S-II, sensor-II; RF, arginine finger. Magnesium ions (Mg2+) are shown as green spheres. The vanadium ion of the vanadate molecule (Van) is shown as a pink sphere.
Extended Data Figure 5 Changes in conformation within dynein AAA+ ring.
a, Superimposition of the AAA1L domains of dynein-2:ADP.Vi (blue) and dynein-1:ADP (grey) shows that helices H2 and H3 of AAA1L are displaced when the H2-β hairpin insert of AAA2L (red) comes into contact with H2 of AAA1L. b, An alignment of the AAA1L domains of dynein-2:ADP.Vi (blue), dynein-1:APO (PDB accession number 4AKI) (pale yellow) and dynein-1:ADP (PDB accession number 3VKG) (grey) shows that the loop containing the sensor-I residue is highly variable between the structures. In the presence of ADP.Vi the loop makes contacts (purple spheres) with AAA2L. c, d, Superimposition of AAA2-AAA4 domains between dynein-2:ADP.Vi and dynein-1:ADP (c) or dynein-1:APO (d) shows that AAA2–AAA4 move as a rigid body.
Extended Data Figure 6 Linker interaction with the AAA+ ring in dynein-2:ADP.Vi, dynein-1:ADP and dynein-1:APO.
a–c, Link3–4 interacts with AAA1L in all structures similar. Mainly hydrophobic contacts exist between the linker H11 helix and the H2 helix as well as the S2 β-sheet of AAA1L. In addition the long peptide that connects the linker with AAA1 (yellow) mediates contacts between Link3–4 and AAA1L. d, Link1–2 is stabilized by contacts with AAA2 and AAA3 in dynein-2:ADP.Vi Link1–2, e by contacts with the AAA2 H2 insert in dynein-1:ADP and f by contacts with AAA5 in dynein-1:APO. Red spheres represent contacts.
Extended Data Figure 7 Conservation of contact sites between linker and dynein ring.
Multiple alignment of cytoplasmic dynein-1 (Cyt-1), dynein-2 (Cyt-2), axonemal inner arm dyneins (IDA) and outer arm γ (ODAg) and αβ (ODAab) dyneins. Dyneins are from human (Hs), Chlamydomonas reinhardtii (Cr), Tetrahymena thermophila (Tt), D. discoideum (Dd), S. cerevisiae (Sc), Drosophila melanogaster (Dm), Emericella nidulans (En) and Candida albicans (Ca). Residues are shaded by conservation, with dark blue being the most conserved. Red asterisks mark hydrophobic contacts that stabilize the bent linker conformation, black asterisks mark the contact site between AAA2L H2 insert (E2028) and the linker (R1413) and green asterisks mark poorly conserved contacts between the linker and the AAA3 H2-S3 insert.
Extended Data Figure 8 Characterization of dynein-2 mutants by negative electron microscopy and microtubule gliding assays.
a, Representative micrographs showing the quality of the raw electron microscopy data. Scale bar, 20 nm. b, Left, histograms showing distribution of angles between the linker and the stalk in three replicate negative-stain electron microscopy experiments (10° bin width); right, representative subclasses used for angle measurement. c, Mean velocities of dynein-2 mutants in microtubule gliding assays. GFP–dynein-2D1091–Q4307 (wild type: WT) glides microtubules at 134 ± 8 nm s−1 (N = 99). The microtubule gliding velocities for the other constructs are ΔAAA3L H2–S3, 59 ± 4 nm s−1 (N = 79); K1413A + E2028A, 49 ± 2 nm s−1 (N = 31); and ΔAAA4L PS-I, 14 ± 1 nm s−1 (N = 121). Microtubule gliding was not observed in case of ΔAAA2L H2 + PS-I. Error bars, s.e.m.
Extended Data Figure 9 The MTBD in dynein-2 ADP.Vi is in the low microtubule affinity conformation.
a, b, Alignment of dynein-2 ADP.Vi MTBD (pale yellow) with dynein-1 MTBDs (grey) in the low microtubule affinity conformation (PDB accession numbers 3ERR and 3WUQ respectively), and c with a dynein-1 MTBD in the high microtubule affinity conformation (PDB accession number 3J1T). The stalk CC1 and the MTBD H1 undergo conformational changes depending on the microtubule affinity of the MTBD. In dynein-2:ADP.Vi the arrangement of these structural elements suggests the MTBD is in the low microtubule affinity conformation. Stalk CC1 and MTBD H1 are coloured blue in low-affinity structures and red in high-affinity structures.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion and Supplementary Data 1. (PDF 314 kb)
: Closure of AAA1/AAA2 interface upon ADP.Vi binding to AAA1 active site
The video starts with an open AAA1 nucleotide binding site as in dynein-1:ADP (PDB ID: 3VKG). The morph into the dynein-2:ADP.Vi crystal structure illustrates how AAA2 PS-I and H2 inserts (red) come into contact with AAA1 H2 helix and close the gap between AAA1L (blue) and AAA2L (cyan) subdomains. ADP.Vi bound to AAA1 is shown in stick representation. (MOV 3073 kb)
The remodeling of the linker during the powerstroke priming
The video starts with the post-powerstroke conformation of the linker in dynein-1:ADP (PDB ID: 3VKG). Linker subdomains 1 and 2 (Link1-2 – pink), the hinge helix (H10 – purple) as well as linker subdomains 3 and 4 (Link3-4 – salmon) adopt a straight conformation. The movie shows a transition to the pre-powerstroke conformation of the linker observed in the dynein-2:ADP.Vi crystal structure. The mobile Link1-2 undergoes a rigid-body movement with respect to the static Link3-4. The hinge helix bends to accommodate the movement. (MOV 2086 kb)
Clash of AAA4 PS-I insert with Link1-2 after AAA1 site closure
The video starts with the dynein-1:ADP structure (PDB ID: 3VKG) where the linker is in the straight post-powerstroke conformation (Link1-2 – pink, hinge helix H10 – purple, Link3-4 – salmon) and morphs into the dynein-2:ADP.Vi structure where the linker is in the bent pre-powerstroke conformation. The closure of the AAA1 nucleotide binding site located at the interface between AAA1 (blue) and AAA2 (cyan) causes a rigid-body movement of AAA2-AAA4 (cyan-green-yellow). The AAA2-AAA4 movement leads to a clash between the AAA4L PS-I insert and Link1-2. The clash is relieved by Link1-2 switching into the pre-powerstroke conformation. (MOV 3417 kb)
The rotation of AAA6L/AAA5S is linked to conformational changes in the stalk/buttress interface
The video starts with the AAA5-AAA6-AAA1 section of the AAA+ ring in the post-powerstroke state as observed in the dynein-1:ADP structure (PDB ID: 3VKG). The central β-sheet of AAA6L (red) points towards AAA1L (pale blue) and both helices of the stalk (yellow) supercoil each other. This view morphs into the pre-powerstroke state as observed in the dynein-2:ADP.Vi structure. AAA6L (red) and AAA5S (orange) rotate as a unit so that the central β-sheet of AAA6L now points towards the AAA+ ring center. The buttress, which extends from AAA5S (orange), slides along the stalk (yellow). The movement of the buttress is correlated with the appearance of a kink in the stalk CC2 helix and a sliding movement of the stalk CC2 relative to the stalk CC1. For clarity other AAA+ domains are shown in pale colours (AAA5L – pale orange, AAA6S – pale red). (MOV 3158 kb)
Conformational changes within the dynein AAA+ ring upon ADP.Vi binding to AAA1
The video starts with dynein-1:ADP AAA+ ring and morphs into the AAA+ ring as observed in dynein-2:ADP.Vi. The linker has been removed for clarity. The ADP.Vi induced closure of the AAA1 site, located at the interface between AAA1 (blue) and AAA2 (cyan), leads to a rigid-body movement of AAA2-AAA4 (cyan-green-yellow). This movement pushes against the AAA5L/AAA4S (orange/yellow) unit which in turn causes the AAA6L/AAA5S (red/orange) unit to rotate. The rotation of AAA6L/AAA5S is linked to the sliding of the coiled-coil helices in the stalk (yellow). (MOV 3201 kb)
Conformational changes within the dynein AAA+ ring are coupled to linker movement
The video displays the same conformational changes as described in Supplementary Movie 5 and in addition shows the remodeling of the linker (Link1-2 – pink, hinge helix H10 – purple, Link3-4 – salmon). AAA1 site closure upon ADP.Vi binding leads to a rigid-body movement of AAA2-AAA4 (cyan-green-yellow) that causes a clash between the AAA4L PS-I insert (yelllow) and Link1-2 (pink). The clash is relieved by Link1-2 switching from the post-powerstroke into the pre-powerstroke conformation. (MOV 2617 kb)
Rights and permissions
About this article
Cite this article
Schmidt, H., Zalyte, R., Urnavicius, L. et al. Structure of human cytoplasmic dynein-2 primed for its power stroke. Nature 518, 435–438 (2015). https://doi.org/10.1038/nature14023
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14023
This article is cited by
-
Integrative proteogenomic characterization of early esophageal cancer
Nature Communications (2023)
-
Regulatory mechanisms of the dynein-2 motility by post-translational modification revealed by MD simulation
Scientific Reports (2023)
-
Chemical fuels for molecular machinery
Nature Chemistry (2022)
-
Optical control of fast and processive engineered myosins in vitro and in living cells
Nature Chemical Biology (2021)
-
Pac1/LIS1 stabilizes an uninhibited conformation of dynein to coordinate its localization and activity
Nature Cell Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.