Abstract
Mast cells are primary effectors in allergic reactions, and may have important roles in disease by secreting histamine and various inflammatory and immunomodulatory substances1,2. Although they are classically activated by immunoglobulin (Ig)E antibodies, a unique property of mast cells is their antibody-independent responsiveness to a range of cationic substances, collectively called basic secretagogues, including inflammatory peptides and drugs associated with allergic-type reactions1,3. The pathogenic roles of these substances have prompted a decades-long search for their receptor(s). Here we report that basic secretagogues activate mouse mast cells in vitro and in vivo through a single receptor, Mrgprb2, the orthologue of the human G-protein-coupled receptor MRGPRX2. Secretagogue-induced histamine release, inflammation and airway contraction are abolished in Mrgprb2-null mutant mice. Furthermore, we show that most classes of US Food and Drug Administration (FDA)-approved peptidergic drugs associated with allergic-type injection-site reactions also activate Mrgprb2 and MRGPRX2, and that injection-site inflammation is absent in mutant mice. Finally, we determine that Mrgprb2 and MRGPRX2 are targets of many small-molecule drugs associated with systemic pseudo-allergic, or anaphylactoid, reactions; we show that drug-induced symptoms of anaphylactoid responses are significantly reduced in knockout mice; and we identify a common chemical motif in several of these molecules that may help predict side effects of other compounds. These discoveries introduce a mouse model to study mast cell activation by basic secretagogues and identify MRGPRX2 as a potential therapeutic target to reduce a subset of drug-induced adverse effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Metcalfe, D. D., Baram, D. & Mekori, Y. A. Mast cells. Physiol. Rev. 77, 1033–1079 (1997)
Galli, S. J., Nakae, S. & Tsai, M. Mast cells in the development of adaptive immune responses. Nature Immunol. 6, 135–142 (2005)
Lagunoff, D., Martin, T. W. & Read, G. Agents that release histamine from mast cells. Annu. Rev. Pharmacol. Toxicol. 23, 331–351 (1983)
Halpern, B. N. & Wood, D. R. The action of promethazine (phenergan) in protecting mice against death due to histamine. Br. J. Pharmacol. Chemother. 5, 510–516 (1950)
Taneike, T., Miyazaki, H., Oikawa, S. & Ohga, A. Compound 48/80 elicits cholinergic contraction through histamine release in the chick oesophagus. Gen. Pharmacol. 19, 689–695 (1988)
Ferry, X., Brehin, S., Kamel, R. & Landry, Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides 23, 1507–1515 (2002)
Purcell, W. M., Doyle, K. M., Westgate, C. & Atterwill, C. K. Characterisation of a functional polyamine site on rat mast cells: association with a NMDA receptor macrocomplex. J. Neuroimmunol. 65, 49–53 (1996)
Tatemoto, K. et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 349, 1322–1328 (2006)
Sick, E., Niederhoffer, N., Takeda, K., Landry, Y. & Gies, J. P. Activation of CD47 receptors causes histamine secretion from mast cells. Cell. Mol. Life Sci. 66, 1271–1282 (2009)
Robas, N., Mead, E. & Fidock, M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J. Biol. Chem. 278, 44400–44404 (2003)
Subramanian, H., Gupta, K., Guo, Q., Price, R. & Ali, H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J. Biol. Chem. 286, 44739–44749 (2011)
Kashem, S. W. et al. G protein coupled receptor specificity for C3a and compound 48/80-induced degranulation in human mast cells: roles of Mas-related genes MrgX1 and MrgX2. Eur. J. Pharmacol. 668, 299–304 (2011)
Subramanian, H. et al. β-Defensins activate human mast cells via Mas-related gene X2. J. Immunol. 191, 345–352 (2013)
Kamohara, M. et al. Identification of MrgX2 as a human G-protein-coupled receptor for proadrenomedullin N-terminal peptides. Biochem. Biophys. Res. Commun. 330, 1146–1152 (2005)
Liu, Q. et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365 (2009)
Mousli, M., Hugli, T. E., Landry, Y. & Bronner, C. Peptidergic pathway in human skin and rat peritoneal mast cell activation. Immunopharmacology 27, 1–11 (1994)
Pundir, P. & Kulka, M. The role of G protein-coupled receptors in mast cell activation by antimicrobial peptides: is there a connection? Immunol. Cell Biol. 88, 632–640 (2010)
Hausmann, O., Schnyder, B. & Pichler, W. J. Etiology and pathogenesis of adverse drug reactions. Chem. Immunol. Allergy 97, 32–46 (2012)
Lumry, W. R. et al. Randomized placebo-controlled trial of the bradykinin B2 receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann. Allergy Asthma Immunol. 107, 529–537 (2011)
Nel, L. & Eren, E. Peri-operative anaphylaxis. Br. J. Clin. Pharmacol. 71, 647–658 (2011)
Read, G. W. Compound 48–80. Structure-activity relations and poly-THIQ, a new, more potent analog. J. Med. Chem. 16, 1292–1295 (1973)
Mertes, P. M., Alla, F., Trechot, P., Auroy, Y. & Jougla, E. Anaphylaxis during anesthesia in France: an 8-year national survey. J. Allergy Clin. Immunol. 128, 366–373 (2011)
Koppert, W. et al. Different patterns of mast cell activation by muscle relaxants in human skin. Anesthesiology 95, 659–667 (2001)
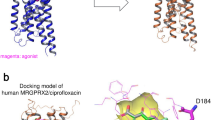
Kelesidis, T., Fleisher, J. & Tsiodras, S. Anaphylactoid reaction considered ciprofloxacin related: a case report and literature review. Clin. Ther. 32, 515–526 (2010)
Blanca-Lopez, N. et al. Hypersensitivity reactions to fluoroquinolones: analysis of the factors involved. Clin. Exp. Allergy 43, 560–567 (2013)
Mori, K., Maru, C. & Takasuna, K. Characterization of histamine release induced by fluoroquinolone antibacterial agents in-vivo and in-vitro. J. Pharm. Pharmacol. 52, 577–584 (2000)
Mori, K., Maru, C., Takasuna, K. & Furuhama, K. Mechanism of histamine release induced by levofloxacin, a fluoroquinolone antibacterial agent. Eur. J. Pharmacol. 394, 51–55 (2000)
Doyle, E., Trosien, J. & Metz, M. Protocols for the induction and evaluation of systemic anaphylaxis in mice. Methods Mol. Biol. 1032, 133–138 (2013)
Bergman, R. K. & Munoz, J. Efficacy of β-adrenergic blocking agents in inducing histamine sensitivity in mice. Nature 217, 1173–1174 (1968)
Matsumura, Y., Tan, E. M. & Vaughan, J. H. Hypersensitivity to histamine and systemic anaphylaxis in mice with pharmacologic β adrenergic blockade: protection by nucleotides. J. Allergy Clin. Immunol. 58, 387–394 (1976)
Han, L. et al. A subpopulation of nociceptors specifically linked to itch. Nature Neurosci. 16, 174–182 (2013)
Siraganian, R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal. Biochem. 57, 383–394 (1974)
Weigand, L. A., Myers, A. C., Meeker, S. & Undem, B. J. Mast cell-cholinergic nerve interaction in mouse airways. J. Physiol. (Lond.) 587, 3355–3362 (2009)
Maurer, M. et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature 432, 512–516 (2004)
Acknowledgements
We thank C. Hawkins and H. Wellington for their assistance in generating MrgprB2-null mice and MrgprB2-Cre BAC transgenic mice. We thank B. Xiao for making MrgprB2-Cre BAC mice and Y. Geng for mouse genotyping. This work was supported by National Institutes of Health grants (R01NS054791 and R01GM087369 to X.D.). X.D. is an Early Career Scientist of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
B.D.M. conceived the project, designed and performed all experiments except where noted, and wrote the paper. P.P. performed all LAD2 cell work with supervision from M.K. S.M. performed tracheal contraction and tissue histamine release experiments. L.H. assisted with BAC purification and staining techniques. B.J.U. supervised S.M. and contributed to experimental design. X.D. supervised the project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 MRGPRX1 orthologues are not expressed at relevant levels in mast cells under naive conditions.
a, Results from a low-stringency RT–PCR screen (see Methods) in peritoneal mast cells for expression of the MRGPRX1 orthologues Mrgpra3 and Mrgprc11. Arrow points to expected band sizes. b, Percentages of peritoneal mast cells responding to the MRGPRX1 and Mrgprc11 agonist bovine adrenal medulla derived peptide, fragment 8–22 (BAM8–22, 500 nM). Activation was assayed by measuring rises in intracellular calcium, using imaging of the Fluo-4 dye. Differences are not significant (P = 0.39). n = 3 mice from each genotype. Group data are expressed as mean ± s.e.m. Two-tailed unpaired Student’s t-test was used to determine significance in statistical comparisons. WT, wild type; KO, knockout. c, Chart summarizing responses to MRGPRX2 ligands and the MRGPRX1 ligand chloroquine (CQ) by HEK293 cells transiently transfected with plasmids driving expression of MRGPRX2, Mrgprb2 and other mouse Mrgpr proteins (that is, Mrgprb1, Mrgprb10 and Mrgprb11) most closely related to Mrgprb2. Positive and negative responses are indicated with ticks and crosses, respectively. Responses were considered positive if at least half of the transfected cells showed a 50% increase in [Ca2+]i. No cells transfected with Mrgprb1, Mrgprb10 and Mrgprb11 responded to any listed drug.
Extended Data Figure 2 Basic secretagogues and drugs that induce pseudo-allergic reactions activate mouse Mrgprb2 and human MRGPRX2 expressed in HEK293 cells.
a, b, Example traces showing changes in [Ca2+]i, as measured by Fluo-4 imaging, from HEK293 cells expressing Mrgprb2 and Gα15 (a) or MRGPRX2 and Gα15 (b). Substances were perfused from the 30 to 90 s time period, except for ciprofloxacin, which was perfused between the 30 and 60 s time periods to minimize exposure to the low pH solutions it was dissolved in. Insulin was used as a negative control. c, Table of half-maximum effective concentration (EC50) values of basic secretagogues and drugs associated with pseudo-allergic reactions to activate Mrgprb2- and MRGPRX2-expressing HEK293 cells. The EC50 values were determined from dose–response studies, which were repeated three times. Data are expressed as mean ± s.e.m.
Extended Data Figure 3 Multiple lines of BAC transgenic mice confirm mast-cell-specific MrgprB2 expression.
Representative confocal images from two other BAC transgenic mouse lines. BAC mice expressing eGFP-Cre in the Mrgprb2 open reading frame were mated to tdTomato reporter mice and tdTomato (red) expression was compared to avidin staining (green), a marker for mast cells. Scale bar, 20 µm.
Extended Data Figure 4 Mrgprb2 is not expressed in mucosal mast cells or peripheral white blood cells.
a, Representative images of a stomach section from an Mrgprb2-tdTomato mouse stained with an anti-MCPT1 (β-chymase) antibody to label mucosal mast cells. White arrows indicate positive cells. No cells were double-labelled (296 Mcpt1-labelled cells and 275 tdTomato-positive cells counted, n = 3 mice). Scale bar, 40 µm. b, Representative images of a Cytospin preparation of peripheral white blood cells from an Mrgprb2-tdTomato mouse doubly labelled with tdTomato for Mrgprb2-expressing cells (red; left image) and Hoechst 33342 nuclear staining (blue; right image). No peripheral white blood cell expressed tdTomato (n = 3 mice; >4,000 cells examined). Scale bar, 40 µm.
Extended Data Figure 5 Mrgprb2MUT mice are functional knockouts.
a, Illustration of the genomic region in and around the Mrgprb2 locus. Note that repetitive sequences including long interspersed elements (LINEs), short interspersed elements (SINEs), and long tandem repeats (LTRs) begin immediately after the 3′ side of the Mrgprb2 gene, and in addition are present within 2.5 kb of the 5′ side. A BLASTN search in March 2014 using the 500 bases adjacent to the 3′ end of Mrgprb2 as a query turned up more than 269,000 hits in the mouse genome. b, Comparison of the wild-type (WT) and mutant (MUT) genomic sequences shows the location of the 4 bp deletion in the mutant. Numbers correspond to the Mrgprb2 open reading frame. c, Sequencing result from wild-type and mutant complementary DNA sampled from mice born 18 months after the mutant line was established. The bases missing in the mutant are highlighted in red. d, Amino acid translation of the Mrgprb2MUT open reading frame reveals that the deletion creates a frameshift mutation and an early termination codon (marked with an asterisk) shortly after the first transmembrane region. Mut, site of the frameshift deletion; TM1, transmembrane region 1.
Extended Data Figure 6 The mast cell numbers and the histamine content of tracheal and skin tissue was not different between wild-type and Mrgprb2MUT animals.
a, Top, representative pictures of avidin staining in wild-type (WT) and Mrgprb2MUT (MUT) mice. Scale bar, 40 µm. Bottom, quantification of mast cell numbers in various tissues. Differences are not significant, using a two-tailed unpaired Student’s t-test (n = 3 mice for each genotype; over 3,000 µm2 and 1,000 µm2 counted for each genotype for hairy and glabrous skin, respectively; over 10,000 peritoneal cells counted). b, The tracheal histamine content averaged 5.9 ± 0.9 and 5.5 ± 1.6 ng mg−1 (n = 5 for each genotype), respectively; the skin histamine content averaged 30.8 ± 3.2 and 30.2 ± 4.0 ng mg−1 (n = 8 for each genotype), respectively. Differences were not significant. Group data are expressed as mean ± s.e.m. Two-tailed unpaired Student’s t-test was used to determine significance in statistical comparisons.
Extended Data Figure 7 Endothelin acting through the ETA GPCR34 induced comparable activation in Mrgprb2MUT and wild-type mast cells.
a, Representative heat map images of mouse peritoneal mast cells showing changes in [Ca2+]i, as assayed by Fluo-4 imaging, induced by bath application of endothelin (1 µM). Scale bar, 10 µm. b, Averages of [Ca2+]i imaging traces for wild-type (WT) (red line) and Mrgprb2MUT (MUT) (black line). The [Ca2+]i traces are similar between wild-type and mutant groups. Traces were averaged as described for Fig. 2a. c, Quantification of percentage of responding cells. Group data are expressed as mean ± s.e.m. Two-tailed unpaired Student’s t-test was used to determine significance in statistical comparisons (n = 3 for each genotype; over 180 cells counted for each genotype). Endothelin-induced responses were not significantly different.
Extended Data Figure 8 IgE-mediated inflammation does not differ between wild-type and MrgprB2MUT mice.
a, Representative images of Evans blue stained extravasation 15 min after intraplantar injection of anti-IgE antibody (right, arrow, 100 µg ml−1, 7 µl in saline) or saline (left). b, Quantification of Evans blue leakage into the paw after 15 min (n = 6 for wild type (WT), n = 7 for MrgprB2MUT(MUT)). Differences after anti-IgE antibody (P = 0.49) and saline (P = 0.23) injection are not significant. Group data are expressed as mean ± s.e.m. Two-tailed unpaired Student’s t-test was used to determine significance in statistical comparisons.
Extended Data Figure 9 Mrgprb2MUT mast cells are unresponsive to basic secretagogues and various therapeutic drugs.
a, Example traces showing changes in [Ca2+]i, as measured by Fluo-4 imaging, from wild-type (WT) and Mrgprb2MUT (MUT) peritoneal mast cells induced by the basic secretagogues from Fig. 2e. Each trace is a response from a unique cell. b, Representative Fluo-4 images (left) and fluorescence traces (right) from wild-type (top) and Mrgprb2MUT (bottom) cultured peritoneal mast cells during application of icatibant (50 µg ml−1). c, Example traces showing changes in [Ca2+]i, as measured by Fluo-4 imaging, from wild-type and Mrgprb2MUT peritoneal mast cells induced by selected FDA-approved cationic peptidergic drugs. Each trace is a response from a unique cell. d, Representative Fluo-4 images (left) and fluorescence traces (right) from wild-type (top) and Mrgprb2MUT (bottom) cultured peritoneal mast cells during application of atracurium (50 µg ml−1). e, Representative Fluo-4 images (left) and fluorescence traces (right) from wild-type (top) and Mrgprb2MUT (bottom) cultured peritoneal mast cells during application of ciprofloxacin (200 µg ml−1).
Extended Data Figure 10 Human mast cells are activated by basic secretagogues and drugs associated with pseudo-allergic reactions in an MRGPRX2-dependent manner.
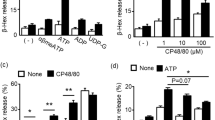
a, Human LAD2 mast cells were treated with different concentrations of compound 48/80, mastoparan, icatibant, atracurium and ciprofloxacin. The activation of mast cells in response to these substances was characterized by the release of β-hexosaminidase, TNF, PGD2 and histamine. In addition, 0.1 µg ml−1 streptavidin stimulation of biotin-conjugated human IgE-sensitized LAD2 cells caused a robust release of β-hexosaminidase (71.3 ± 1.8% release), compared with untreated cells (4.1 ± 0.3% release). Group data are expressed as mean ± s.e.m. b, Knockdown of human MRGPRX2 significantly reduced mast cell activation evoked by basic secretagogues and drugs associated with pseudo-allergic reactions, but not by IgE. Human LAD2 mast cells were first transfected with siRNA against MRGPRX2 or control siRNA. Two days after the transfection, the cells were treated with compound 48/80 (0.1 µg ml−1), mastoparan (5 µg ml−1), icatibant (10 µg ml−1), atracurium (25 µg ml−1) and ciprofloxacin (75 µg ml−1). The activation of mast cells in response to these substances characterized by the release of β-hexosaminidase was significantly reduced in MRGPRX2-siRNA-treated cells, compared to release in the control group. IgE-mediated mast cell degranulation was unaffected by MRGPRX2 siRNA knockdown. Group data are expressed as mean ± s.e.m. Two-tailed unpaired Student’s t-test was used to determine significance in statistical comparisons, and differences were considered significant at *P < 0.05, **P < 0.01, ***P < 0.005 (the experiments were repeated three times).
Supplementary information
Supplementary Information
This file contains a Supplementary Table and Chart and additional references. (PDF 541 kb)
Rights and permissions
About this article
Cite this article
McNeil, B., Pundir, P., Meeker, S. et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241 (2015). https://doi.org/10.1038/nature14022
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14022
This article is cited by
-
Proinflammatory chemokine CXCL14 activates MAS-related G protein-coupled receptor MRGPRX2 and its putative mouse ortholog MRGPRB2
Communications Biology (2024)
-
Immunopathogenesis of urticaria: a clinical perspective on histamine and cytokine involvement
Inflammation Research (2024)
-
Pseudo-allergic reactions induced by Chinese medicine injections: a review
Chinese Medicine (2023)
-
Compound 48/80 increases murine bladder wall compliance independent of mast cells
Scientific Reports (2023)
-
New perspectives on the origins and heterogeneity of mast cells
Nature Reviews Immunology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.