Abstract
During the long Sahelian dry season, mosquito vectors of malaria are expected to perish when no larval sites are available; yet, days after the first rains, mosquitoes reappear in large numbers. How these vectors persist over the 3–6-month long dry season has not been resolved, despite extensive research for over a century1,2,3. Hypotheses for vector persistence include dry-season diapause (aestivation) and long-distance migration (LDM); both are facets of vector biology that have been highly controversial owing to lack of concrete evidence. Here we show that certain species persist by a form of aestivation, while others engage in LDM. Using time-series analyses, the seasonal cycles of Anopheles coluzzii, Anopheles gambiae sensu stricto (s.s.), and Anopheles arabiensis were estimated, and their effects were found to be significant, stable and highly species-specific. Contrary to all expectations, the most complex dynamics occurred during the dry season, when the density of A. coluzzii fluctuated markedly, peaking when migration would seem highly unlikely, whereas A. gambiae s.s. was undetected. The population growth of A. coluzzii followed the first rains closely, consistent with aestivation, whereas the growth phase of both A. gambiae s.s. and A. arabiensis lagged by two months. Such a delay is incompatible with local persistence, but fits LDM. Surviving the long dry season in situ allows A. coluzzii to predominate and form the primary force of malaria transmission. Our results reveal profound ecological divergence between A. coluzzii and A. gambiae s.s., whose standing as distinct species has been challenged, and suggest that climate is one of the selective pressures that led to their speciation. Incorporating vector dormancy and LDM is key to predicting shifts in the range of malaria due to global climate change4, and to the elimination of malaria from Africa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Donnelly, M. J., Simard, F. & Lehmann, T. Evolutionary studies of malaria vectors. Trends Parasitol. 18, 75–80 (2002)
Omer, S. M. & Cloudsley-Thompson, J. L. Dry season biology of Anopheles gambiae Giles in the Sudan. Nature 217, 879–880 (1968)
Holstein, M. H. Biology of Anopheles gambiae. Research in French West Africa (World Health Organization, 1954)
Siraj, A. S. et al. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science 343, 1154–1158 (2014)
World Health Organization. World Malaria Report 2013 (World Health Organization, 2013)
Coetzee, M. et al. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 3619, 246–274 (2013)
Omer, S. M. & Cloudsley-Thompson, J. L. Survival of female Anopheles gambiae Giles through a 9-month dry season in Sudan. Bull. World Health Organ. 42, 319–330 (1970)
Taylor, C. E., Toure, Y. T., Coluzzi, M. & Petrarca, V. Effective population size and persistence of Anopheles arabiensis during the dry season in west Africa. Med. Vet. Entomol. 7, 351–357 (1993)
Touré, Y. T. et al. Ecological genetic studies in the chromosomal form Mopti of Anopheles gambiae s.s. in Mali, West Africa. Genetica 94, 213–223 (1994)
Simard, F., Lehmann, T., Lemasson, J. J., Diatta, M. & Fontenille, D. Persistence of Anopheles arabiensis during the severe dry season conditions in Senegal: an indirect approach using microsatellite loci. Insect Mol. Biol. 9, 467–479 (2000)
Coetzee, M., Craig, M. & le Sueur, D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol. Today 16, 74–77 (2000)
della Torre, A., Tu, Z. & Petrarca, V. On the distribution and genetic differentiation of Anopheles gambiae s. s. molecular forms. Insect Biochem. Mol. Biol. 35, 755–769 (2005)
Sogoba, N. et al. Monitoring of larval habitats and mosquito densities in the Sudan savanna of Mali: implications for malaria vector control. Am. J. Trop. Med. Hyg. 77, 82–88 (2007)
Adamou, A. et al. The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar. J. 10, 151 (2011)
Huestis, D. L. et al. Seasonal variation in metabolic rate, flight activity and body size of Anopheles gambiae in the Sahel. J. Exp. Biol. 215, 2013–2021 (2012)
Lehmann, T. et al. Aestivation of the African malaria mosquito, Anopheles gambiae in the Sahel. Am. J. Trop. Med. Hyg. 83, 601–606 (2010)
Yaro, A. S. et al. Dry season reproductive depression of Anopheles gambiae in the Sahel. J. Insect Physiol. 58, 1050–1059 (2012)
Lehmann, T. et al. Seasonal variation in spatial distributions of Anopheles gambiae in a Sahelian village: evidence for aestivation. J. Med. Entomol. 51, 27–38 (2014)
Coluzzi, M., Petrarca, V. & Di Deco, M. A. Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae. Boll. Zool. 52, 45–63 (1985)
Coluzzi, M., Sabatini, A., Petrarca, V. & Di Deco, M. A. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 73, 483–497 (1979)
Sogoba, N. et al. Spatial distribution of the chromosomal forms of Anopheles gambiae in Mali. Malar. J. 7, 205 (2008)
Toure, Y. T. et al. Perennial transmission of malaria by the Anopheles gambiae complex in a north Sudan Savanna area of Mali. Med. Vet. Entomol. 10, 197–199 (1996)
Denlinger, D. L. Dormancy in tropical insects. Annu. Rev. Entomol. 31, 239–264 (1986)
Denlinger, D. L. & Armbruster, P. A. Mosquito diapause. Annu. Rev. Entomol. 59, 73–93 (2014)
Tauber, M. J., Tauber, C. A. & Masaki, S. Seasonal Adaptations of Insects (Oxford Univ. Press, 1986)
Sogoba, N. et al. Malaria transmission dynamics in Niono, Mali: the effect of the irrigation systems. Acta Trop. 101, 232–240 (2007)
Lemasson, J. J. et al. Comparison of behavior and vector efficiency of Anopheles gambiae and An. arabiensis (Diptera:Culicidae) in Barkedji, a Sahelian area of Senegal. J. Med. Entomol. 34, 396–403 (1997)
Huestis, D. L. & Lehmann, T. Ecophysiology of Anopheles gambiae s. l.: persistence in the Sahel. Infect. Genet. Evol. http://dx.doi.org/10.1016/j.meegid.2014.05.027 (14 June 2014)
Lehmann, T. & Diabate, A. The molecular forms of Anopheles gambiae: a phenotypic perspective. Infect. Genet. Evol. 8, 737–746 (2008)
SAS (Sas Institute, Cary, North Carolina, 2011)
Fanello, C., Santolamazza, F. & della Torre, A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med. Vet. Entomol. 16, 461–464 (2002)
Harvey, A. C. Forecasting, Structural Time Series Models and the Kalman Filter (Cambridge Univ. Press, 1989)
Acknowledgements
We thank the residents of Thierola for their hospitality and assistance with mosquito collections; J. Ribeiro, T. Wellems, P. McQueen, R. Faiman and G. Wasserberg for their comments on previous versions of this manuscript; and C. Traoré, R. Sakai, R. Gwadz and T. Wellems for logistical support. This study was supported by the Tamaki Foundation and by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Author information
Authors and Affiliations
Contributions
T.L. conceived the study and together with A.D. and A.S.Y. designed it. A.D., A.S.Y., M.D., S.T., D.L.H., Y.K., A.I.T., Z.L.S. and D.S. performed the research, both in the field and the laboratory. All authors have discussed and interpreted the results as well as made decisions on various field and laboratory operations. A.D. led the field operations and data management; T.L. analysed the data and wrote the paper, with extensive input from A.D. and D.L.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Sex-ratio, density and composition of Anopheles gambiae s.l.
a–c, Overall monthly means of the proportion of A. gambiae s.l. females (a), house density (b), and species composition (c). Nm, Nd, and Ng denote sample size of A. gambiae s.l., the number of collection days, and the number genotyped to species, respectively (Methods). Whiskers in box-whisker plots extend to the extreme values up to 1.5 the distance between the twenty-fifth and seventy-fifth percentiles. In a, blue triangles represent means that are significantly lower than the red triangles (based on the sequential Bonferroni test; see Extended Data Table 1) and the horizontal line represents 1:1 sex ratio.
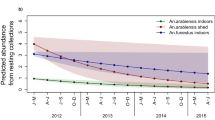
Extended Data Figure 2 Population dynamics of Anopheles gambiae s.l.
House density over time in linear (top) and natural logarithm (bottom) scale to evaluate systematic change over time. Circles denote observed daily mean density and the black lines show the interpolated series of mean house density over 5-day intervals. Grey lines depict interpolation during the longest time without field samples (December 2008 to April 2009). First rain events are shown by green lines (dates listed above). Sample sizes of mosquitoes and collection days are the same as in Extended Data Fig. 1b.
Extended Data Figure 3 Population dynamics of Anopheles gambiae s.l. across years.
Observed daily mean density (circles) is shown against 5-day means (line) on linear and log scales from July to June of every year to assess similarity among years. Green arrows mark the first rain and the tan background denotes the dry season. Shading during 2008 indicates a gap in sampling (December–March) when imputed values were used (Methods). Sample sizes (Nm and Nd) are explained in Fig. 1.
Extended Data Figure 4 Level-adjusted seasonal component of the density of A. coluzzii, A. gambiae s.s. and A. arabiensis.
The level, seasonals, and their 95% CI (bands) were estimated using unobserved component time series models (Table 1; Methods). Arrows denote the start of the population growth (text and Fig. 2). Time is shown starting from May to maximize the comparability between the species. In the colour ruler on the x axis, yellow and green denote dry and wet seasons, respectively, and orange and light-green denote transition periods. Sample sizes are given in Extended Data Fig. 1.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion and Supplementary references. (PDF 211 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Dao, A., Yaro, A., Diallo, M. et al. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nature 516, 387–390 (2014). https://doi.org/10.1038/nature13987
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13987
This article is cited by
-
Genomics reveals heterogeneous Plasmodium falciparum transmission and selection signals in Zambia
Communications Medicine (2024)
-
The impact of pyrethroid-pyriproxyfen and pyrethroid-chlorfenapyr long-lasting insecticidal nets on density of primary malaria vectors Anopheles gambiae s.s. and Anopheles coluzzii in Benin: a secondary analysis of a cluster randomised controlled trial
Parasites & Vectors (2024)
-
Potential persistence mechanisms of the major Anopheles gambiae species complex malaria vectors in sub-Saharan Africa: a narrative review
Malaria Journal (2023)
-
Molecular drivers of insecticide resistance in the Sahelo-Sudanian populations of a major malaria vector Anopheles coluzzii
BMC Biology (2023)
-
Stability of a Vector-Borne Disease Model with a Delayed Nonlinear Incidence
Acta Applicandae Mathematicae (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.