Abstract
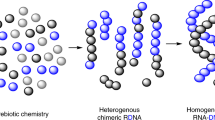
The emergence of catalysis in early genetic polymers such as RNA is considered a key transition in the origin of life1, pre-dating the appearance of protein enzymes. DNA also demonstrates the capacity to fold into three-dimensional structures and form catalysts in vitro2. However, to what degree these natural biopolymers comprise functionally privileged chemical scaffolds3 for folding or the evolution of catalysis is not known. The ability of synthetic genetic polymers (XNAs) with alternative backbone chemistries not found in nature to fold into defined structures and bind ligands4 raises the possibility that these too might be capable of forming catalysts (XNAzymes). Here we report the discovery of such XNAzymes, elaborated in four different chemistries (arabino nucleic acids, ANA5; 2′-fluoroarabino nucleic acids, FANA6; hexitol nucleic acids, HNA; and cyclohexene nucleic acids, CeNA7) directly from random XNA oligomer pools, exhibiting in trans RNA endonuclease and ligase activities. We also describe an XNA–XNA ligase metalloenzyme in the FANA framework, establishing catalysis in an entirely synthetic system and enabling the synthesis of FANA oligomers and an active RNA endonuclease FANAzyme from its constituent parts. These results extend catalysis beyond biopolymers and establish technologies for the discovery of catalysts in a wide range of polymer scaffolds not found in nature8. Evolution of catalysis independent of any natural polymer has implications for the definition of chemical boundary conditions for the emergence of life on Earth and elsewhere in the Universe9.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Atkins J. F., Gesteland R. F., Cech T. R., eds. RNA Worlds (Cold Spring Harbor Laboratory, 2012)
Breaker, R. R. & Joyce, G. F. A DNA enzyme that cleaves RNA. Chem. Biol. 1, 223–229 (1994)
Eschenmoser, A. Chemical etiology of nucleic acid structure. Science 284, 2118–2124 (1999)
Pinheiro, V. B. et al. Synthetic genetic polymers capable of heredity and evolution. Science 336, 341–344 (2012)
Noronha, A. M. et al. Synthesis and biophysical properties of arabinonucleic acids (ANA): circular dichroic spectra, melting temperatures, and ribonuclease H susceptibility of ANA·RNA hybrid duplexes. Biochemistry 39, 7050–7062 (2000)
Wilds, C. J. 2′-Deoxy-2′-fluoro-β-d-arabinonucleosides and oligonucleotides (2′F-ANA): synthesis and physicochemical studies. Nucleic Acids Res. 28, 3625–3635 (2000)
Herdewijn, P. Nucleic acids with a six-membered ‘carbohydrate’ mimic in the backbone. Chem. Biodivers. 7, 1–59 (2010)
Pinheiro, V. B. & Holliger, P. The XNA world: progress towards replication and evolution of synthetic genetic polymers. Curr. Opin. Chem. Biol. 16, 245–252 (2012)
Benner, S. A., Ricardo, A. & Carrigan, M. A. Is there a common chemical model for life in the universe? Curr. Opin. Chem. Biol. 8, 672–689 (2004)
Valadkhan, S. & Manley, J. L. Splicing-related catalysis by protein-free snRNAs. Nature 413, 701–707 (2001)
Nissen, P., Hansen, J., Ban, N., Moore, P. B. & Steitz, T. A. The structural basis of ribosome activity in peptide bond synthesis. Science 289, 920–930 (2000)
Joyce, G. F. Directed evolution of nucleic acid enzymes. Annu. Rev. Biochem. 73, 791–836 (2004)
Martín-Pintado, N. et al. The solution structure of double helical arabino nucleic acids (ANA and 2′F-ANA): effect of arabinoses in duplex-hairpin interconversion. Nucleic Acids Res. 40, 9329–9339 (2012)
Lilley, D. M. J. Mechanisms of RNA catalysis. Phil. Trans. R. Soc. Lond. B 366, 2910–2917 (2011)
Wilkinson, K. A., Merino, E. J. & Weeks, K. M. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): quantitative RNA structure analysis at single nucleotide resolution. Nature Protocols 1, 1610–1616 (2006)
Homan, P. J. et al. Single-molecule correlated chemical probing of RNA. Proc. Natl Acad. Sci. USA 111, 13858–13863 (2014)
Santoro, S. W. & Joyce, G. F. A general purpose RNA-cleaving DNA enzyme. Proc. Natl Acad. Sci. USA 94, 4262–4266 (1997)
Santoro, S. W. & Joyce, G. F. Mechanism and utility of an RNA-cleaving DNA enzyme. Biochemistry 37, 13330–13342 (1998)
Minasov, G., Teplova, M., Nielsen, P., Wengel, J. & Egli, M. Structural basis of cleavage by RNase H of hybrids of arabinonucleic acids and RNA. Biochemistry 39, 3525–3532 (2000)
Bartel, D. P. & Szostak, J. W. Isolation of new ribozymes from a large pool of random sequences [see comment]. Science 261, 1411–1418 (1993)
Paul, N., Springsteen, G. & Joyce, G. F. Conversion of a ribozyme to a deoxyribozyme through in vitro evolution. Chem. Biol. 13, 329–338 (2006)
Ekland, E. H., Szostak, J. W. & Bartel, D. P. Structurally complex and highly active RNA ligases derived from random RNA sequences. Science 269, 364–370 (1995)
Rogers, J. & Joyce, G. F. The effect of cytidine on the structure and function of an RNA ligase ribozyme. RNA 7, 395–404 (2001)
Cuenoud, B. & Szostak, J. W. A. DNA metalloenzyme with DNA ligase activity. Nature 375, 611–614 (1995)
Lescrinier, E. et al. Solution structure of an HNA-RNA hybrid. Chem. Biol. 7, 719–731 (2000)
Dowler, T., Bergeron, D. & Tedeschi, A. L. Improvements in siRNA properties mediated by 2′-deoxy-2′-fluoro-β-d-arabinonucleic acid (FANA). Nucleic Acids Res. 34, 1669–1675 (2006)
Hendrix, C. et al. 1′,5′-Anhydrohexitol oligonucleotides: hybridisation and strand displacement with oligoribonucleotides, interaction with RNase H and HIV reverse transcriptase. Chemistry 3, 1513–1520 (1997)
Nauwelaerts, K., Fisher, M. & Froeyen, M. Structural characterization and biological evaluation of small interfering RNAs containing cyclohexenyl nucleosides. J. Am. Chem. Soc. 129, 9340–9348 (2007)
Deleavey, G. F. et al. Synergistic effects between analogs of DNA and RNA improve the potency of siRNA-mediated gene silencing. Nucleic Acids Res. 38, 4547–4557 (2010)
Zlatev, I., Manoharan, M., Vasseur, J.-J. & Morvan, F. Solid-phase chemical synthesis of 5′-triphosphate DNA, RNA, and chemically modified oligonucleotides. In Current Protocols in Nucleic Acid Chemistry http://dx.doi.org/10.1002/0471142700.nc0128s50 (Wiley & Sons, 2001)
Chu, B. C., Wahl, G. M. & Orgel, L. E. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 11, 6513–6529 (1983)
Brody, J. R. & Kern, S. E. Sodium boric acid: a Tris-free, cooler conductive medium for DNA electrophoresis. Biotechniques 36, 214–216 (2004)
Goecks, J., Nekrutenko, A. & Taylor, J. &. The Galaxy Team. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 11, R86 (2010)
Blankenberg, D. et al. Galaxy: a web-based genome analysis tool for experimentalists. In Current Protocols in Molecular Biology Ch. 19, Unit 19.10.1–21. (2010)
Giardine, B. et al. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 15, 1451–1455 (2005)
Bowler, F. R. et al. Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation. Nature Chem. 5, 383–389 (2013)
Siegfried, N. A., Busan, S., Rice, G. M., Nelson, J. A. E. & Weeks, K. M. RNA motif discovery by SHAPE and mutational profiling (SHAPE-MaP). Nature Methods 11, 959–965 (2014)
Mortimer, S. A. & Weeks, K. M. A. Fast-acting reagent for accurate analysis of RNA secondary and tertiary structure by SHAPE chemistry. J. Am. Chem. Soc. 129, 4144–4145 (2007)
Hajdin, C. E. et al. Accurate SHAPE-directed RNA secondary structure modeling, including pseudoknots. Proc. Natl Acad. Sci. USA 110, 5498–5503 (2013)
Lorenz, R. et al. ViennaRNA Package 2.0. Algorithms Mol. Biol. 6, 26 (2011)
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003)
Acknowledgements
This work was supported by the Medical Research Council (MRC) programme grant U105178804 (P. Holliger, A.I.T., V.B.P., A.S.M., S.P.-C., C.C.) and by grants from the European Science Foundation (ESF) and the Biotechnology and Biological Sciences Research Council (BBSRC) UK (09-EuroSYNBIO-OP-013) (P.H., A.I.T.), the European Union Framework (FP7/2007-2013 (P. Herdewijn), the European Research Council (ERC-2012 ADG_20120216/320683 (P. Herdewijn)), the US National Science Foundation (MCB-1121024 (K.M.W.)) and by an NSF Graduate Research Fellowship (DGE-1144081 (M.J.S.)).
Author information
Authors and Affiliations
Contributions
A.I.T. and P. Holliger conceived and designed the experiments. A.I.T. performed XNAzyme selections and characterized XNAzymes with V.B.P., A.S.M., S.P.-C., C.C. V.B.P. generated the improved HNA synthetase. M.J.S. and K.M.W. performed and analysed SHAPE and DMS mapping experiments. P. Herdewijn synthesized hNTPs, ceNTPs and aGTP. All authors analysed data and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Selection scheme for RNA endonuclease XNAzymes.
a, XNA library preparation using DNA-dependent XNA polymerases, templated using N40temp, N10_817spike_temp, or N10_1023spike_temp DNA oligonucleotides (see Supplementary Table 1) primed by a biotinylated chimaeric DNA–RNA primer (NucPrim), which serves as substrate for RNA cleavage in cis. Libraries are captured by streptavidin beads, allowing denaturation and removal of DNA templates. b, Single-stranded libraries are annealed and incubated in reaction buffer (see Methods), successful XNAzymes cleave the biotinylated RNA substrate in cis. c, Size separation of reacted XNA pools using denaturing polyacrylamide electrophoresis (urea–PAGE). Cleaved XNA pools are gel-extracted (indicated by dashed box) and incubated with streptavidin beads in order to capture and discard any uncleaved carry-over (indicated by dashed arrow). d, Reverse transcription of isolated, cleaved XNA pools using XNA-dependent DNA polymerase RT521L (that is, XNA → cDNA). e, Amplification of transcribed cDNA by successive PCR reactions, using the primers indicated (see Supplementary Table 1). f, PCR reaction generating templates for XNA synthesis for further rounds of selection. See Methods for details. Solid crosses indicate removal of denatured strands using streptavidin bead capture.
Extended Data Figure 2 Sequences of RNA endonuclease XNAzymes.
a, Schematic diagram showing DNA–RNA-(red)–XNA(purple) chimaeric library setup for selection of in cis RNA-cleaving XNAzymes. The sequences of the XNA region under selection (dashed box) of the most abundant clones revealed by deep sequencing are shown for selections using b, FANA, c, ANA, d, HNA, and e, CeNA. The top 10 sequences, or representatives of sequence families, were screened by Urea-PAGE gel shift for activity in cis (unimolecular reaction, as selected) and in trans (bimolecular reaction). Sequences chosen for further characterization are indicated by coloured boxes.
Extended Data Figure 3 Sequence dependence of RNA endonuclease XNAzyme cleavage.
XNAzymes were selected with degeneracy in the RNA substrate (see Extended Data Fig. 2a). The sequence requirements at these positions (upstream of the cleavage sites shown by a black inverted triangle) in the RNA substrate (N10 and N11 shown in red) were determined by urea–PAGE gel shift using all 16 variants of the substrate NucSR with each XNAzyme in trans: a, FR17_6 (FANA), b, AR17_5 (ANA), c, HR16_1 (HNA), d, CeR16_3 (CeNA). e, RNA substrate NucSR_AG (lane 1) was reacted in trans with RNA endonuclease DNAzyme 8-1717 synthesized as DNA (lane 2), HNA (lane 3), CeNA (lane 4), FANA (lane 5) or ANA (lane 6). Activity of 8-17 is lost upon conversion to the XNAs described. f, Sequence requirements for RNA cleavage by FANAzyme FR17_6 min proximal to the cleavage site (black inverted triangle), positions 8 and 9 (highlighted in red) of minimized RNA substrate NucSR_min. Substrate sequences used for further characterization of each XNAzyme are indicated by an asterisk.
Extended Data Figure 4 Analysis of RNA endonuclease XNAzyme cleavage products.
a, 5′ cleavage product of FANAzyme FR17_6 reaction shows expected mass for a 2′,3′ cyclic phosphate (>p) using matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-ToF). b, Hydrolysis of 5′ FR17_6 cleavage product >p in low pH and dephosphosphorylation with calf intestinal phosphatase (removes 2′p or 3′p, but not >p). c, Phosphorylation of 3′ FR17_6 cleavage product with T4 polynucleotide kinase (adds 5′p). Mass spectra and dephosphorylation assays of 5′ cleavage products of d, ANAzyme AR17_5, e, HNAzyme HR16_1 and f, CeNAzyme CeR16_3 reveal all RNA endonuclease XNAzymes yield products with 2′,3′ cyclic phosphates. (RNase T1) and (−OH) indicate partial hydrolysis reactions of the RNA substrates used. g, Bivalent metal ion requirements and titration of, h, pH or i, MgCl2, of FANAzyme FR17_6min reaction with NucSR_min. j, Reaction catalysed by RNA endonuclease XNAzymes.
Extended Data Figure 5 Chemical probing of XNAzyme secondary structures.
a, Chemical probing using selective 2′hydroxyl acylation analysed by primer extension (SHAPE)(RNA)15 or, b, d, dimethyl sulphate (DMS)16 (RNA and FANA) footprinting, used to inform secondary structure predictions of c, RNA endonuclease FANAzyme FR17_6 or e, FANA ligase FANAzyme FpImR4_2, embedded in structural cassettes15, with RNA substrate (inactivated by a 2′O-methyl modification at position 11, indicated by an asterisk in all panels) or FANA product in cis. SHAPE analyses RNA 2′OH. Black, orange and red solid bars and bases indicate low, moderate, and high SHAPE reactivity, respectively, for positions 1-28 in FR17_6 construct, corresponding to RNA substrate NucSR. DMS reacts predominantly with A and C bases (RNA and FANA). Positions were defined as reactive (blue open bars and circles) if reactivity was greater than a cut-off (dashed line in b and d) of one half standard deviation above the median. Dashed circles indicate positions with marginal reactivity. Site of cleavage (in unmodified RNA) or ligation is indicated by a black inverted triangle. Primer-binding regions (no structural data) are shown in grey.
Extended Data Figure 6 Selection scheme for RNA ligase XNAzymes.
a, XNA library preparation using DNA-dependent XNA polymerases, primed by a 5′ triphosphorylated (5′ppp) RNA primer (LigS2R), which serves as one of the substrates for RNA ligation in cis. Libraries are synthesized with 3′ biotinylated DNA templates (N40temp(b); see Supplementary Table 1), allowing subsequent capture and removal by streptavidin beads. b, Single-stranded libraries (unbiotinylated) are annealed and incubated in reaction buffer (see Methods) together with a biotinylated chimaeric DNA–RNA substrate (tag1_LigS1R), which successful XNAzymes ligate to RNA substrate LigS2R in cis. c, Size separation of reacted XNA pools using urea–PAGE. Ligated XNA pools are gel-extracted (indicated by dashed box). d, Reverse transcription of XNA pools using XNA-dependent DNA polymerase RT521L, which is also able to transcribe RNA across the ligation junction (that is, [RNA–RNA–XNA] → cDNA). e, Amplification of transcribed cDNA by successive PCR reactions, using the primers indicated (see Supplementary Table 1); out-nest reaction depends on priming site (tag1) from ligated substrate tag1_LigS1R. f, PCR reaction generating templates for XNA synthesis (now 5′ biotinylated) for further rounds of selection. Solid crosses indicate removal of denatured strands using streptavidin bead capture.
Extended Data Figure 7 Sequences and analyses of RNA ligase XNAzymes.
a, Schematic diagram showing RNA (red)–XNA (purple) chimaeric library setup for selection of FANAzymes capable of catalysing a bimolecular RNA ligation. b, Sequences of the FANA region under selection (dashed box in a) of the most abundant clones revealed by deep sequencing. Representatives of sequence families were screened for activity in bimolecular (LigS2R attached to XNAzyme) or trimolecular (XNAzyme separate from both substrates) reactions. Sequence F2R17_1 (boxed) was chosen for further characterization. c, Regiospecificity of RNA product (LigPR) of ligation catalysed by XNAzyme F2R17_1min (see Fig. 3), analysed by strong anion exchange chromatography (SAX-HPLC)36. Mock RNA ligation product (i–iii) containing a single 2′-5′ (Mock_LigPR[2′-5′]) or 3′-5′ linkage (Mock_LigPR[3′-5′]) at a position analogous to the ligation site were compared to the XNAzyme-catalysed RNA product LigPR (iv-vi). The XNAzyme product gives an identical elution profile to the natural (3′-5′) linkage standard. d, Bivalent metal ion requirements and titration of, e, pH or f, MgCl2, of FANAzyme F2R17_1min reaction. g, Substitution of RNA ligase substrates with DNA and XNA (FANA) versions in F2R17_1min reaction shows that 5′-RNA–RNA-3′ ligation is preferred, but some ligase activity can be seen with 5′-RNA–DNA-3′.
Extended Data Figure 8 Selection scheme for XNA ligase XNAzymes.
a, XNA library preparation using DNA-dependent XNA polymerases, primed by an all-XNA (FANA) primer (LigS2F), which serves as one of the substrates for FANA ligation in cis. Libraries are synthesized with 3′ biotinylated DNA template (E47spike_temp; see Supplementary Table 1), allowing subsequent capture and removal by streptavidin beads. b, Single-stranded libraries (unbiotinylated) are annealed and incubated in reaction buffer (see Methods) together with a biotinylated chimaeric DNA–XNA (FANA) substrate (tag1_LigS1F), activated with a 3′ phosphorylimidazolide (Extended Data Fig. 10), which successful XNAzymes ligate to XNA (FANA) substrate LigS2F in cis. c, Size separation of reacted XNA pools using urea–PAGE. Ligated XNA pools are gel-extracted (indicated by dashed box). d, Reverse transcription of XNA pools using XNA-dependent DNA polymerase RT521L (that is, XNA → cDNA). e, Amplification of transcribed cDNA by successive PCR reactions, using the primers indicated (see Supplementary Table 1); out-nest reaction depends on priming site (tag1) from ligated substrate tag1_LigS1F. f, PCR reaction generating templates for XNA synthesis (now 5′ biotinylated) for further rounds of selection. Solid crosses indicate removal of denatured strands using streptavidin bead capture.
Extended Data Figure 9 Sequences and analyses of XNA ligase XNAzymes (FANA).
a, Schematic diagram showing all-FANA library setup for selection of FANAzymes capable of catalysing a bimolecular XNA (FANA) ligation. b, FANA sequences of the region under selection (dashed box in a) of the most abundant clones revealed by deep sequencing. Representatives of sequence families were screened for activity in bimolecular (LigS2F attached to XNAzyme) or trimolecular (XNAzyme separate from both substrates) reactions. Sequence FpImR4_2 (boxed) was chosen for further characterization. c, Regiospecificity of XNA (FANA) product (LigPF) of ligation catalysed by XNAzyme FpImR4_2 (see Fig. 4), analysed by strong anion exchange chromatography (SAX-HPLC). Mock FANA ligation product (Mock_LigPF) (i), prepared by polymerase (D4K) gives an identical elution profile to the XNAzyme-catalysed FANA product (LigPF)(ii and iii). d, Bivalent metal ion requirements and titration of, e, pH or f, MgCl2, of FANAzyme FpImR4_2 reaction. g, Substitution of XNA ligase substrates with RNA and DNA versions in FpImR4_2 reaction. Although 5′-FANA–FANA-3′ is preferred, ligase activity can be seen with 5′-FANA–DNA-3′, and 5′-FANA–RNA-3′, as well as 5′-DNA–FANA-3′, 5′-DNA–DNA-3′ and 5′-DNA–RNA-3′.
Extended Data Figure 10 Analysis of XNA (FANA) substrates and enzymes prepared by solid-phase synthesis.
MALDI-ToF mass spectra showing expected masses of a, XNA (FANA) ligase substrate LigS1F-3′phosphorylimidazolide (prepared by solid-phase synthesis of the 3′ phosphorylated (3′p) oligonucleotide, followed by reaction with carbodiimide and imidazole (see Methods)), b, XNA (FANA) ligase substrate LigS2F, and XNAzymes c, FR17_6min, d, F2R17_6min, and e, FpImR4_2. f, Dephosphorylation assay of versions of LigS1F (3′ hydroxyl, lanes 1 and 2, 3′ phosphate, lanes 2 and 3, or 3′ phorphorylimidazolide, lanes 5 and 6) with calf intestinal phosphatase (lanes 2, 4 and 6). The majority of the LigS1F preparation shown (∼70%) is protected from dephosphorylation, consistent with formation of the 3′pIm. g, Urea–PAGE analyses of purified FANA substrates and XNAzymes.
Supplementary information
Supplementary Tables
This file contains Supplementary Table 1. (PDF 122 kb)
Rights and permissions
About this article
Cite this article
Taylor, A., Pinheiro, V., Smola, M. et al. Catalysts from synthetic genetic polymers. Nature 518, 427–430 (2015). https://doi.org/10.1038/nature13982
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13982
This article is cited by
-
Functionalization of acyclic xenonucleic acid with modified nucleobases
Polymer Journal (2023)
-
A two-residue nascent-strand steric gate controls synthesis of 2′-O-methyl- and 2′-O-(2-methoxyethyl)-RNA
Nature Chemistry (2023)
-
An RNA-cleaving threose nucleic acid enzyme capable of single point mutation discrimination
Nature Chemistry (2022)
-
XNAzymes targeting the SARS-CoV-2 genome inhibit viral infection
Nature Communications (2022)
-
Evaluation of 3′-phosphate as a transient protecting group for controlled enzymatic synthesis of DNA and XNA oligonucleotides
Communications Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.