Abstract
The role of cellular metabolism in regulating cell proliferation and differentiation remains poorly understood1. For example, most mammalian cells cannot proliferate without exogenous glutamine supplementation even though glutamine is a non-essential amino acid1,2. Here we show that mouse embryonic stem (ES) cells grown under conditions that maintain naive pluripotency3 are capable of proliferation in the absence of exogenous glutamine. Despite this, ES cells consume high levels of exogenous glutamine when the metabolite is available. In comparison to more differentiated cells, naive ES cells utilize both glucose and glutamine catabolism to maintain a high level of intracellular α-ketoglutarate (αKG). Consequently, naive ES cells exhibit an elevated αKG to succinate ratio that promotes histone/DNA demethylation and maintains pluripotency. Direct manipulation of the intracellular αKG/succinate ratio is sufficient to regulate multiple chromatin modifications, including H3K27me3 and ten-eleven translocation (Tet)-dependent DNA demethylation, which contribute to the regulation of pluripotency-associated gene expression. In vitro, supplementation with cell-permeable αKG directly supports ES-cell self-renewal while cell-permeable succinate promotes differentiation. This work reveals that intracellular αKG/succinate levels can contribute to the maintenance of cellular identity and have a mechanistic role in the transcriptional and epigenetic state of stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011)
Eagle, H., Oyama, V. I., Levy, M., Horton, C. L. & Fleischman, R. The growth response of mammalian cells in tissue culture to l-glutamine and l-glutamic acid. J. Biol. Chem. 218, 607–616 (1956)
Ying, Q. L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008)
Nichols, J., Silva, J., Roode, M. & Smith, A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136, 3215–3222 (2009)
Smith, Z. D. et al. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 484, 339–344 (2012)
Wray, J., Kalkan, T. & Smith, A. G. The ground state of pluripotency. Biochem. Soc. Trans. 38, 1027–1032 (2010)
Leitch, H. G. et al. Naive pluripotency is associated with global DNA hypomethylation. Nature Struct. Mol. Biol. 20, 311–316 (2013)
Ficz, G. et al. FGF signaling inhibition in ESCs drives rapid genome-wide demethylation to the epigenetic ground state of pluripotency. Cell Stem Cell 13, 351–359 (2013)
Habibi, E. et al. Whole-genome bisulfite sequencing of two distinct interconvertible DNA methylomes of mouse embryonic stem cells. Cell Stem Cell 13, 360–369 (2013)
Borgel, J. et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nature Genet. 42, 1093–1100 (2010)
Marks, H. et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149, 590–604 (2012)
Ying, Q. L., Nichols, J., Chambers, I. & Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281–292 (2003)
Zhang, J., Nuebel, E., Daley, G. Q., Koehler, C. M. & Teitell, M. A. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 11, 589–595 (2012)
Kaelin, W. G., Jr Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb. Symp. Quant. Biol. 76, 335–345 (2011)
Cloos, P. A., Christensen, J., Agger, K. & Helin, K. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev. 22, 1115–1140 (2008)
Kruidenier, L. et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature 488, 404–408 (2012)
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006)
Boyer, L. A. et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349–353 (2006)
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007)
Blaschke, K. et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 500, 222–226 (2013)
Hackett, J. A. et al. Synergistic mechanisms of DNA demethylation during transition to ground-state pluripotency. Stem Cell Reports 1, 518–531 (2013)
Faddah, D. A. et al. Single-cell analysis reveals that expression of Nanog is biallelic and equally variable as that of other pluripotency factors in mouse ESCs. Cell Stem Cell 13, 23–29 (2013)
Shipony, Z. et al. Dynamic and static maintenance of epigenetic memory in pluripotent and somatic cells. Nature 513, 115–119 (2014)
Markoulaki, S., Meissner, A. & Jaenisch, R. Somatic cell nuclear transfer and derivation of embryonic stem cells in the mouse. Methods 45, 101–114 (2008)
Dawlaty, M. M. et al. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev. Cell 24, 310–323 (2013)
Buganim, Y. et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 150, 1209–1222 (2012)
Millard, P., Letisse, F., Sokol, S. & Portais, J. C. IsoCor: correcting MS data in isotope labeling experiments. Bioinformatics 28, 1294–1296 (2012)
Yuan, J., Bennett, B. D. & Rabinowitz, J. D. Kinetic flux profiling for quantitation of cellular metabolic fluxes. Nature Protocols 3, 1328–1340 (2008)
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959)
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nature Protocols 8, 2281–2308 (2013)
Acknowledgements
B.W.C. is a Howard Hughes Medical Institute fellow of the Jane Coffin Childs Memorial Research Fund. L.W.S.F. is the Jack Sorrell Fellow of the Damon Runyon Cancer Research Foundation (DRG-2144-13). This work was funded by grants from the National Institutes of Health/National Institute of General Medical Sciences (C.D.A.) and from the National Cancer Institute (C.B.T.). We thank C. Li for assistance with FACS analysis and M. Dawlaty, D. Faddah and R. Jaenisch for sharing cell lines used in this study.
Author information
Authors and Affiliations
Contributions
B.W.C. and L.W.S.F. designed and performed all experiments in the study under the guidance of C.D.A. and C.B.T. J.R.C. contributed material support. B.W.C., L.W.S.F., C.D.A. and C.B.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
C.B.T. is a co-founder of Agios Pharmaceuticals and a member of the board directors of Merck. C.D.A. is a co-founder of Chroma Therapeutics and Constellation Pharmaceuticals.
Extended data figures and tables
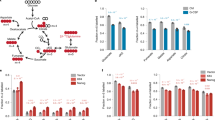
Extended Data Figure 1 Pluripotent stem cells can proliferate in the absence of glutamine when cultured in 2i/LIF medium.
a, Doubling time of ESC-V19 cells cultured in S/L or 2i/L. b, Growth curve of ESC-1 cells cultured in S/L or S/L/2i medium devoid of glucose. c, Samples of S/L (left) and 2i/L (right) media with and without glutamine were analysed by GC–MS. Representative chromatograms of the total ion count reveal a clear glutamine (Q) peak in +Q media (grey) and no detectable glutamine in −Q media (red). m, minutes. d, Teratoma formation from ES cells grown in 2i/L medium without glutamine for 3 days. Representative images of haematoxylin and eosin staining reveal neural tissue (ectoderm), hepatocytes and pancreatic acinar cells (endoderm) and smooth muscle (mesoderm). Scale bar, 200 μm. e, Growth curve of ESC-1 cells grown in glutamine-free 2i/L or 2i medium. f, Gene expression analysis confirms that EpiSCs, which represent post-implantation pluripotency, were generated from ESC-1 cells by culture with Fgf and activin A. Transcript levels were assessed by quantitative real-time polymerase chain reaction with reverse transcription (qRT–PCR), normalized to Gapdh and expressed as a ratio of values of mouse ES cells cultured in 2i/L medium. g, Growth curve of EpiSCs cultured in serum-free epiblast medium (serum-free medium containing FGF and activin A (Fgf/ActA)) with or without glutamine. h, Growth curve of an iPSC line derived from fibroblasts using Oct3/4 (O), Klf4 (K) and Sox2 (S) cultured in glutamine-free S/L or 2i/L medium. i, Doubling time of ESC-1 cells cultured in 2i/L medium in the presence and absence of glutamine. j, Growth curve of ESC-V19 cells cultured in glutamine-free 2i/L medium in the presence or absence of 1 mM methylsulphoxide (MSO). k, ESC-V19 cells grown in glutamine-free S/L (left) or 2i/L (right) medium with or without 4 mM DM-αKG. For growth curve experiments, cells were seeded on day 0 in complete medium and then were changed to experimental medium on day 1 (indicated by red arrow). Data are presented as the mean ± s.d. of triplicate wells from a representative experiment.
Extended Data Figure 2 Mouse ES cells cultured with 2i demonstrate altered glucose and glutamine utilization.
a, 2i enables glutamate synthesis from glucose-derived carbons. ESC-1 cells cultured in S/L, S/L/2i or 2i/L medium were incubated with medium containing [U-13C]glucose for 4 h and the fraction of glutamate containing glucose-derived carbons is shown. b, ESC-1 cells were cultured for 4 h in glutamine-free S/L or 2i/L medium containing [U-13C]glucose and the total amount of glutamate labelled by glucose-derived carbons is shown. c, Incorporation of 14C derived from [U-14C]glucose (14C-glc) (left) or derived from [U-14C]glutamine (14C-gln) (right) into total cellular protein after 48 h incubation. P < 0.05 for 14C-glc, P = 0.1 for 14C-gln, calculated by unpaired two-tailed Student’s t-test. Data are presented as the mean ± s.d. of triplicate wells (a, b) or ± s.e.m. of quadruplicate wells (c) from a representative experiment.
Extended Data Figure 3 Regulation of histone methylation in 2i/LIF cells.
a, Western blot analysis of ESC-1 cells grown in glutamine-free 2i/L medium for 24 h with supplementation as indicated. b, c, H3K27me3 ChIP-qPCR of ESC-1 cells cultured in S/L (b) or 2i/L (c) medium with or without 30 μM GSK-J4 for 5 h. Data are presented as the mean ± s.e.m. of triplicate samples. *P < 0.05 by unpaired Student’s two-tailed t-test.
Extended Data Figure 4 Generation of JMJD3 mutant cells.
a, Schematic of targeting strategy for guide RNAs (gRNAs) to mouse Jmjd3 exon 17. gRNA sequences are highlighted in blue. b, Representative sequences from two clones used in this study. Sanger sequencing revealed indels as shown in schematic. Red dashes, deleted bases; red bases, insertions. gRNA is highlighted in blue and protospacer adjacent motif (PAM) sequences are identified in green. Predicted cut site is indicated by red triangle. Location of in-frame downstream stop is indicated on the right. c, An example chromatogram for clone JMJD3Δ/Δ-2 showing single-base-pair insertions at the predicted Cas9 cleavage site.
Extended Data Figure 5 αKG increases Tet activity in mouse ES cells.
a, Simplified schematic of the reaction mechanism of Tet1/2 enzymes. b, Relative per cent 5-methylcytosine (% 5-mC) in ESC-1 cells cultured in S/L medium with or without DM-αKG for 24 h. Each data point represents a sample from triplicate wells of a representative experiment. c, Gene expression in ESC-1 cells cultured with DM-αKG or DM-succinate for 3 days. d, Gene expression in ESC-1 cells cultured in S/L medium with or without DM-αKG for four passages. e, Gene expression in wild-type or Tet1/Tet2 double-knockout (KO) mouse ES cells cultured with DM-αKG or DM-succinate for 72 h. qRT–PCR data (c–e) was normalized to actin or Gapdh and samples were normalized to the control group. Oct3/4 is not expected to change and is included as a control. Data are presented as the mean ± s.e.m. of triplicate wells.
Extended Data Figure 6 αKG increases Nanog expression.
a, Representative histogram of GFP intensity of Nanog–GFP cells treated with or without DM-αKG for 3 days. Grey represents background staining. b, ESC-1 cells were cultured in S/L medium with DM-αKG for four passages and then switched to medium containing the indicated amounts of DM-αKG (0.5–4 mM) or vehicle control (S/L) for 3 days. GFP expression (M.F.I.) was determined by fluorescence-activated cell sorting (FACS). Data are presented as the mean ± s.d. of triplicate wells from a representative experiment.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion and Supplementary References. (PDF 151 kb)
Rights and permissions
About this article
Cite this article
Carey, B., Finley, L., Cross, J. et al. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 (2015). https://doi.org/10.1038/nature13981
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13981
This article is cited by
-
Inhibition of fatty acid oxidation enables heart regeneration in adult mice
Nature (2023)
-
TGF-β1 is a regulator of the pyruvate dehydrogenase complex in fibroblasts
Scientific Reports (2020)
-
CD44 regulates epigenetic plasticity by mediating iron endocytosis
Nature Chemistry (2020)
-
Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations
Nature Communications (2020)
-
Glutamine reliance in cell metabolism
Experimental & Molecular Medicine (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.