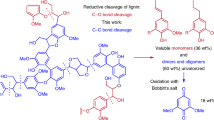
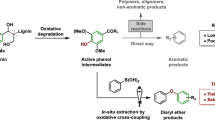
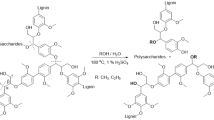
Abstract
Lignin is a heterogeneous aromatic biopolymer that accounts for nearly 30% of the organic carbon on Earth1 and is one of the few renewable sources of aromatic chemicals2. As the most recalcitrant of the three components of lignocellulosic biomass (cellulose, hemicellulose and lignin)3, lignin has been treated as a waste product in the pulp and paper industry, where it is burned to supply energy and recover pulping chemicals in the operation of paper mills4. Extraction of higher value from lignin is increasingly recognized as being crucial to the economic viability of integrated biorefineries5,6. Depolymerization is an important starting point for many lignin valorization strategies, because it could generate valuable aromatic chemicals and/or provide a source of low-molecular-mass feedstocks suitable for downstream processing7. Commercial precedents show that certain types of lignin (lignosulphonates) may be converted into vanillin and other marketable products8,9, but new technologies are needed to enhance the lignin value chain. The complex, irregular structure of lignin complicates chemical conversion efforts, and known depolymerization methods typically afford ill-defined products in low yields (that is, less than 10–20wt%)2,10,11. Here we describe a method for the depolymerization of oxidized lignin under mild conditions in aqueous formic acid that results in more than 60wt% yield of low-molecular-mass aromatics. We present the discovery of this facile C–O cleavage method, its application to aspen lignin depolymerization, and mechanistic insights into the reaction. The broader implications of these results for lignin conversion and biomass refining are also considered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ralph, J., Brunow, G. & Boerjan, W. Lignins. eLShttp://dx.doi.org/10.1002/9780470015902.a0020104 (2007)
Zakzeski, J., Bruijnincx, P. C. A., Jongerius, A. L. & Weckhuysen, B. M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010)
Sanderson, K. Lignocellulose: a chewy problem. Nature 474, S12–S14 (2011)
Lin, S. Y. & Lin, I. S. in Ullmann’s Encyclopedia of Industrial Chemistry 5th edn (eds Elvers, B., Hawkins, S., Schulz, G. ) Vol. A15 305–315 (VCH, 1985)
Tuck, C. O., Pérez, E., Horváth, I. T., Sheldon, R. A. & Poliakoff, M. Valorization of biomass: deriving more value from waste. Science 337, 695–699 (2012)
Holladay, J. E., Bozell, J. J., White, J. F. & Johnson, D. Top Value-added Chemicals from Biomass Vol. 2 http://www1.eere.energy.gov/bioenergy/pdfs/pnnl-16983.pdf (United States Department of Energy, 2007)
Ragauskas, A. J. et al. Lignin valorization: improving lignin processing in the biorefinery. Science 344, 1246843 (2014)
Hocking, M. B. Vanillin: synthetic flavoring from spent sulfite liquor. J. Chem. Educ. 74, 1055–1059 (1997)
Business Areas. Borregaard http://www.borregaard.com/Business-Areas (20 June 2014)
Pandey, M. P. & Kim, C. S. Lignin depolymerization and conversion: a review of thermochemical methods. Chem. Eng. Technol. 34, 29–41 (2011)
Wang, H., Tucker, M. & Ji, Y. Recent development in chemical depolymerization of lignin: a review. J. Appl. Chem 2013, 1–9 (2013)
Gierer, J. & Norén, I. Oxidative pretreatment of pine wood to facilitate delignification during kraft pulping. Holzforschung 36, 123–130 (1982)
Ljunggren, S. & Olsson, A. The specificity in oxidation of some lignin and carbohydrate models and pine wood shavings with permanganate and pyridinum dichloromate before the kraft pulping process. Holzforschung 38, 91–99 (1984)
Rahimi, A., Azarpira, A., Kim, H., Ralph, J. & Stahl, S. S. Chemoselective metal-free aerobic oxidation in lignin. J. Am. Chem. Soc. 135, 6415–6418 (2013)
Crestini, C. & D’Auria, M. Singlet oxygen in the photodegradation of lignin models. Tetrahedron 53, 7877–7888 (1997)
Fukagawa, N. & Ishizu, A. Photoreaction of phenacyl aryl ether type lignols. J. Wood Chem. Technol. 11, 263–289 (1991)
Argyropoulos, D. S. & Sun, Y. Photochemically induced solid-state degradation, condensation, and rearrangement reactions in lignin model compounds and milled wood lignin. Photochem. Photobiol. 64, 510–517 (1996)
Vanucci, C., De Violet, P. F., Bouas-Laurent, H. & Castellan, A. Photodegradation of lignin: a photophysical and photochemical study of a non-phenolic α-carbonyl β-O-4 lignin model dimer, 3,4-dimethoxy-α-(2′-methoxyphenoxy) acetophenone. J. Photochem. Photobiol. Chem. 41, 251–265 (1988)
Nguyen, J. D., Matsuura, B. S. & Stephenson, C. R. J. A photochemical strategy for lignin degradation at room temperature. J. Am. Chem. Soc. 136, 1218–1221 (2014)
Collinson, S. R. & Thielemans, W. The catalytic oxidation of biomass to new materials focusing on starch, cellulose and lignin. Coord. Chem. Rev. 254, 1854–1870 (2010)
Chatel, G. & Rogers, R. D. Oxidation of lignin using ionic liquids—an innovative strategy to produce renewable chemicals. ACS Sustainable Chem. Eng. 2, 322–339 (2014)
Chang, H., Cowling, E. B. & Brown, W. Comparative studies on cellulolytic enzyme lignin and milled wood lignin of sweetgum and spruce. Holzforschung 29, 153–159 (1975)
Ralph, J. & Landucci, L. L. in Lignin and Lignans; Advances in Chemistry (eds Heitner, C., Dimmel, D. R. & Schmidt, J. A. ) 137–234 (CRC Press, 2010)
Fouad, F. M. & Farrell, P. G. Primary and secondary kinetic isotope effects in E2 elimination reactions. Tetrahedr. Lett. 19, 4735–4738 (1978)
Xu, W., Miller, S. J., Agrawal, P. K. & Jones, C. W. Depolymerization and hydrodeoxygenation of switchgrass lignin with formic acid. ChemSusChem 5, 667–675 (2012)
Toledano, A. et al. Fractionation of organosolv lignin from olive tree clippings and its valorization to simple phenolic compounds. ChemSusChem 6, 529–536 (2013)
Bauer, K., Garber, D. & Surburg, H. in Ullmann’s Encyclopedia of Industrial Chemistry 5th edn (eds Elvers, B., Rounsaville, J. F. & Schulz, G. ) Vol. A11 199–200 (VCH, 1985)
Bjørsvik, H.-R. & Liguori, L. Organic processes to pharmaceutical chemicals based on fine chemicals from lignosulfonates. Org. Process Res. Dev. 6, 279–290 (2002)
Martínez, Á. T. et al. Monolignol acylation and lignin structure in some nonwoody plants: a 2D NMR study. Phytochemistry 69, 2831–2843 (2008)
Luterbacher, J. S. et al. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 343, 277–280 (2014)
Acknowledgements
We thank J. Ralph for numerous helpful discussions, H. Kim and A. Azarpira for assistance with the purification and NMR characterization of aspen lignin, and S. Chakraborty for assistance with gel-permeation chromatographic analysis of lignin samples. Financial support for this project was provided by the Great Lakes Bioenergy Research Center (Department of Energy Biological and Environmental Research Office of Science DE-FC02-07ER64494). The NMR facility was partly supported by the National Science Foundation (CHE-9208463) and the National Institutes of Health (S10 RR08389).
Author information
Authors and Affiliations
Contributions
A.R. and S.S.S. conceived the idea for the lignin depolymerisation. A.R. performed the chemical reactions of lignin and lignin model compounds, including NMR spectroscopic characterization of the products. A.U. and J.J.C. designed the analytical approach to characterize the lignin depolymerization products. A.U. performed LC/MS analysis. A.R. and S.S.S. wrote the manuscript in consultation with A.U. and J.J.C.
Corresponding author
Ethics declarations
Competing interests
A patent application based on the results reported here has been submitted.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Figures 1-6 and Supplementary Tables 1-2. (PDF 3606 kb)
Rights and permissions
About this article
Cite this article
Rahimi, A., Ulbrich, A., Coon, J. et al. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515, 249–252 (2014). https://doi.org/10.1038/nature13867
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13867
This article is cited by
-
Carbon–carbon bond cleavage for a lignin refinery
Nature Chemical Engineering (2024)
-
Aqueous amine enables sustainable monosaccharide, monophenol, and pyridine base coproduction in lignocellulosic biorefineries
Nature Communications (2024)
-
An overview of lignin pathways of valorization: from isolation to refining and conversion into value-added products
Biomass Conversion and Biorefinery (2024)
-
Lignin Degradation by Isolated Lignolytic Acinetobacter baumanii S2, Aspergillus niger SF4 and Rhodotorula glutinis and Profiling Products from Bio-Valorization Perspective
Waste and Biomass Valorization (2024)
-
Laccase-catalyzed lignin depolymerization in deep eutectic solvents: challenges and prospects
Bioresources and Bioprocessing (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.