Abstract
The ability to store energy on the electric grid would greatly improve its efficiency and reliability while enabling the integration of intermittent renewable energy technologies (such as wind and solar) into baseload supply1,2,3,4. Batteries have long been considered strong candidate solutions owing to their small spatial footprint, mechanical simplicity and flexibility in siting. However, the barrier to widespread adoption of batteries is their high cost. Here we describe a lithium–antimony–lead liquid metal battery that potentially meets the performance specifications for stationary energy storage applications. This Li||Sb–Pb battery comprises a liquid lithium negative electrode, a molten salt electrolyte, and a liquid antimony–lead alloy positive electrode, which self-segregate by density into three distinct layers owing to the immiscibility of the contiguous salt and metal phases. The all-liquid construction confers the advantages of higher current density, longer cycle life and simpler manufacturing of large-scale storage systems (because no membranes or separators are involved) relative to those of conventional batteries5,6. At charge–discharge current densities of 275 milliamperes per square centimetre, the cells cycled at 450 degrees Celsius with 98 per cent Coulombic efficiency and 73 per cent round-trip energy efficiency. To provide evidence of their high power capability, the cells were discharged and charged at current densities as high as 1,000 milliamperes per square centimetre. Measured capacity loss after operation for 1,800 hours (more than 450 charge–discharge cycles at 100 per cent depth of discharge) projects retention of over 85 per cent of initial capacity after ten years of daily cycling. Our results demonstrate that alloying a high-melting-point, high-voltage metal (antimony) with a low-melting-point, low-cost metal (lead) advantageously decreases the operating temperature while maintaining a high cell voltage. Apart from the fact that this finding puts us on a desirable cost trajectory, this approach may well be more broadly applicable to other battery chemistries.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Soloveichik, G. L. Battery technologies for large-scale stationary energy storage. Annu. Rev. Chem. Biomol. Eng. 2, 503–527 (2011)
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011)
Yang, Z. et al. Electrochemical energy storage for green grid. Chem. Rev. 111, 3577–3613 (2011)
Barnhart, C. J. & Benson, S. M. On the importance of reducing the energetic and material demands of electrical energy storage. Energy Environ. Sci. 6, 1083–1092 (2013)
Kim, H. et al. Liquid metal batteries: past, present, and future. Chem. Rev. 113, 2075–2099 (2013)
Bradwell, D. J., Kim, H., Sirk, A. H. C. & Sadoway, D. R. Magnesium-antimony liquid metal battery for stationary energy storage. J. Am. Chem. Soc. 134, 1895–1897 (2012)
Weppner, W. & Huggins, R. A. Thermodynamic properties of the intermetallic systems lithium-antimony and lithium-bismuth. J. Electrochem. Soc. 125, 7–14 (1978)
Cairns, E. J. et al. Galvanic Cells with Fused-Salt Electrolytes. Tech. Report ANL-7316 (Argonne National Laboratory, 1967)
Eckert, C. A., Irwin, R. B. & Smith, J. S. Thermodynamic activity of magnesium in several highly-solvating liquid alloys. Metall. Trans. B 14, 451–458 (1983)
Morachevskii, A. G., Bochagina, E. V. & Bykova, M. A. Thermodynamic properties of bismuth-sodium-antimony liquid alloys. Zh. Prikl. Khim. 73, 1620–1624 (2011)
Ohtani, H., Okuda, K. & Ishida, K. Thermodynamic study of phase equilibria in the Pb-Sn-Sb System. J. Phase Equilibria 16, 416–429 (1995)
Morachevskii, A. G. Thermodynamic analysis of alloys of the lithium-antimony system. Zh. Prikl. Khim. 75, 367–369 (2002)
Gasior, W. & Moser, Z. Thermodynamic study of lithium-lead alloys using the EMF method. J. Nucl. Mater. 294, 77–83 (2001)
Dworkin, A. S., Bronstein, H. R. & Bredig, M. A. Miscibility of metals with salts. VI. Lithium-lithium halide systems. J. Phys. Chem. 66, 572–573 (1962)
Kanevskii, L. S. & Dubasova, V. S. Degradation of lithium-ion batteries and how to fight it: a review. Russ. J. Electrochem. 41, 1–16 (2005); Elektrokhimiya. 41, 3–19 (2005)
Acknowledgements
We acknowledge financial support from the Advanced Research Projects Agency-Energy (US Department of Energy) and Total SA.
Author information
Authors and Affiliations
Contributions
K.W. and K.J. contributed equally to this work. K.W. and K.J. conducted equilibrium voltage measurements. K.W., K.J., T.O., D.J.B. and U.M. performed small-scale cell testing. B.C. and P.J.B. performed cell testing at engineering scale. D.R.S., D.A.B. and H.K. had the idea for the project. K.W., K.J., B.C., T.O. and D.R.S. drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.J.B. and D.R.S. are co-founders of Ambri, a company established to commercialize the liquid metal battery. D.J.B. is now Chief Technology Officer at Ambri. His contributions to this article were made while he was still a student at MIT pursuing his PhD and subsequently a postdoctoral associate for a short period of time. D.R.S.’s role with the company is advisory; he is formally the Chief Scientific Advisor and is a member of the Board of Directors.
Extended data figures and tables
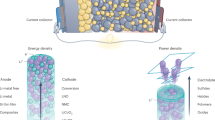
Extended Data Figure 1 Cell schematic of Li||Sb–Pb liquid metal battery.
The negative current collector consists of a stainless steel rod and Fe–Ni foam. The positive current collector is made of graphite (small cell; 3.16 cm2 active area) or 304 stainless steel (large cell; 62 cm2 active area). Current collectors are electrically isolated by means of an alumina insulator.
Rights and permissions
About this article
Cite this article
Wang, K., Jiang, K., Chung, B. et al. Lithium–antimony–lead liquid metal battery for grid-level energy storage. Nature 514, 348–350 (2014). https://doi.org/10.1038/nature13700
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13700
This article is cited by
-
Constructing robust heterostructured interface for anode-free zinc batteries with ultrahigh capacities
Nature Communications (2023)
-
Development of design strategies for conjugated polymer binders in lithium-ion batteries
Polymer Journal (2023)
-
Liquid metal-based textiles for smart clothes
Science China Technological Sciences (2023)
-
Materials, fundamentals, and technologies of liquid metals toward carbon neutrality
Science China Technological Sciences (2023)
-
Electrochemically recycling degraded superalloy and valorizing CO2 in the affordable borate-modified molten electrolyte
Tungsten (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.