Abstract
Eukaryotic circadian oscillators consist of negative feedback loops that generate endogenous rhythmicities1. Natural antisense RNAs are found in a wide range of eukaryotic organisms2,3,4,5. Nevertheless, the physiological importance and mode of action of most antisense RNAs are not clear6,7,8,9. frequency (frq) encodes a component of the Neurospora core circadian negative feedback loop, which was thought to generate sustained rhythmicity10. Transcription of qrf, the long non-coding frq antisense RNA, is induced by light, and its level oscillates in antiphase to frq sense RNA3. Here we show that qrf transcription is regulated by both light-dependent and light-independent mechanisms. Light-dependent qrf transcription represses frq expression and regulates clock resetting. Light-independent qrf expression, on the other hand, is required for circadian rhythmicity. frq transcription also inhibits qrf expression and drives the antiphasic rhythm of qrf transcripts. The mutual inhibition of frq and qrf transcription thus forms a double negative feedback loop that is interlocked with the core feedback loop. Genetic and mathematical modelling analyses indicate that such an arrangement is required for robust and sustained circadian rhythmicity. Moreover, our results suggest that antisense transcription inhibits sense expression by mediating chromatin modifications and premature termination of transcription. Taken together, our results establish antisense transcription as an essential feature in a circadian system and shed light on the importance and mechanism of antisense action.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dunlap, J. C. Molecular bases for circadian clocks. Cell 96, 271–290 (1999)
Berretta, J. & Morillon, A. Pervasive transcription constitutes a new level of eukaryotic genome regulation. EMBO Rep. 10, 973–982 (2009)
Kramer, C., Loros, J. J., Dunlap, J. C. & Crosthwaite, S. K. Role for antisense RNA in regulating circadian clock function in Neurospora crassa. Nature 421, 948–952 (2003)
Nagano, T. et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322, 1717–1720 (2008)
Deuve, J. L. & Avner, P. The coupling of X-chromosome inactivation to pluripotency. Annu. Rev. Cell Dev. Biol. 27, 611–629 (2011)
Camblong, J., Iglesias, N., Fickentscher, C., Dieppois, G. & Stutz, F. Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation in S. cerevisiae. Cell 131, 706–717 (2007)
Ogawa, Y., Sun, B. K. & Lee, J. T. Intersection of the RNA interference and X-inactivation pathways. Science 320, 1336–1341 (2008)
Prescott, E. M. & Proudfoot, N. J. Transcriptional collision between convergent genes in budding yeast. Proc. Natl Acad. Sci. USA 99, 8796–8801 (2002)
Hobson, D. J., Wei, W., Steinmetz, L. M. & Svejstrup, J. Q. RNA polymerase II collision interrupts convergent transcription. Mol. Cell 48, 365–374 (2012)
Heintzen, C. & Liu, Y. The Neurospora crassa circadian clock. Adv. Genet. 58, 25–66 (2007)
Crosthwaite, S. K., Dunlap, J. C. & Loros, J. J. Neurospora wc-1 and wc-2: Transcription, photoresponses, and the origins of circadian rhythmicity. Science 276, 763–769 (1997)
He, Q. et al. White collar-1, a DNA binding transcription factor and a light sensor. Science 297, 840–843 (2002)
Froehlich, A. C., Liu, Y., Loros, J. J. & Dunlap, J. C. White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 297, 815–819 (2002)
Smith, K. M. et al. Transcription factors in light and circadian clock signaling networks revealed by genomewide mapping of direct targets for Neurospora white collar complex. Eukaryot. Cell 9, 1549–1556 (2010)
Aronson, B. D., Johnson, K. A. & Dunlap, J. C. The circadian clock locus frequency: a single ORF defines period length and temperature compensation. Proc. Natl Acad. Sci. USA 91, 7683–7687 (1994)
Gooch, V. D. et al. Fully codon-optimized luciferase uncovers novel temperature characteristics of the Neurospora clock. Eukaryot. Cell 7, 28–37 (2008)
Bell-Pedersen, D., Dunlap, J. C. & Loros, J. J. Distinct cis-acting elements mediate clock, light, and developmental regulation of the Neurospora crassa eas (ccg-2) gene. Mol. Cell. Biol. 16, 513–521 (1996)
Hong, C. I., Jolma, I. W., Loros, J. J., Dunlap, J. C. & Ruoff, P. Simulating dark expressions and interactions of frq and wc-1 in the Neurospora circadian clock. Biophys. J. 94, 1221–1232 (2008)
Chang, S. S., Zhang, Z. & Liu, Y. RNA interference pathways in fungi: mechanisms and functions. Annu. Rev. Microbiol. 66, 305–323 (2012)
Dang, Y., Li, L., Guo, W., Xue, Z. & Liu, Y. Convergent transcription induces dynamic DNA Methylation at disiRNA loci. PLoS Genet. 9, e1003761 (2013)
Belden, W. J., Lewis, Z. A., Selker, E. U., Loros, J. J. & Dunlap, J. C. CHD1 remodels chromatin and influences transient DNA methylation at the clock gene frequency. PLoS Genet. 7, e1002166 (2011)
Hsin, J. P. & Manley, J. L. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 26, 2119–2137 (2012)
Li, B., Howe, L., Anderson, S., Yates, J. R., III & Workman, J. L. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J. Biol. Chem. 278, 8897–8903 (2003)
Adhvaryu, K. K., Morris, S. A., Strahl, B. D. & Selker, E. U. Methylation of histone H3 lysine 36 is required for normal development in Neurospora crassa. Eukaryot. Cell 4, 1455–1464 (2005)
Zhou, Z. et al. Suppression of WC-independent frequency transcription by RCO-1 is essential for Neurospora circadian clock. Proc. Natl Acad. Sci. USA 110, E4867–E4874 (2013)
Guo, J., Cheng, P., Yuan, H. & Liu, Y. The exosome regulates circadian gene expression in a posttranscriptional negative feedback loop. Cell 138, 1236–1246 (2009)
Sauman, I. & Reppert, S. M. Circadian clock neurons in the silkmoth Antheraea pernyi: novel mechanisms of Period protein regulation. Neuron 17, 889–900 (1996)
Koike, N. et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338, 349–354 (2012)
Menet, J. S., Rodriguez, J., Abruzzi, K. C. & Rosbash, M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. eLife 1, e00011 (2012)
Vollmers, C. et al. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab. 16, 833–845 (2012)
Choudhary, S. et al. A double-stranded-RNA response program important for RNA interference efficiency. Mol. Cell. Biol. 27, 3995–4005 (2007)
Zhao, Y. et al. Ubiquitin ligase components Cullin4 and DDB1 are essential for DNA methylation in Neurospora crassa. J. Biol. Chem. 285, 4355–4365 (2010)
Bardiya, N. & Shiu, P. K. Cyclosporin A-resistance based gene placement system for Neurospora crassa. Fungal Genet. Biol. 44, 307–314 (2007)
Liu, Y., Garceau, N., Loros, J. J. & Dunlap, J. C. Thermally regulated translational control mediates an aspect of temperature compensation in the Neurospora circadian clock. Cell 89, 477–486 (1997)
Dharmananda, S. Studies of the Circadian Clock of Neurospora crassa: Light-induced Phase Shifting. PhD thesis, Univ. California, Santa Cruz. (1980)
Barlow, J. J., Mathias, A. P., Williamson, R. & Gammack, D. B. A simple method for the quantitative isolation of undegraded high molecular weight ribonucleic acid. Biochem. Biophys. Res. Commun. 13, 61–66 (1963)
Yoon, O. K. & Brem, R. B. Noncanonical transcript forms in yeast and their regulation during environmental stress. RNA 16, 1256–1267 (2010)
He, Q. & Liu, Y. Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev. 19, 2888–2899 (2005)
Pall, M. L. The use of Ignite (Basta; glufosinate;phosphinothricin) to select transformants of bar-containing plasmids in Neurospora crassa. Fungal Genet. Newsl. 40, 57 (1993)
Ruoff, P., Loros, J. J. & Dunlap, J. C. The relationship between FRQ-protein stability and temperature compensation in the Neurospora circadian clock. Proc. Natl Acad. Sci. USA 102, 17681–17686 (2005)
Ruoff, P., Vinsjevik, M., Monnerjahn, C. & Rensing, L. The Goodwin oscillator: on the importance of degradation reactions in the circadian clock. J. Biol. Rhythms 14, 469–479 (1999)
Yu, Y. et al. A genetic network for the clock of Neurospora crassa. Proc. Natl Acad. Sci. USA 104, 2809–2814 (2007)
Gear, C. Simultaneous numerical solution of differential-algebraic equations. IEEE Trans. Circ. Syst. 18, 89–95 (1971)
Ermentrout, B. XPPAUT. Scholarpedia 2, 1399, http://www.scholarpedia.org/article/XPPAUT (2007)
Acknowledgements
We thank J. Cha, Y. Dang and H. Yuan for technical assistance, and B. Li for critical comments. Supported by grants from the National Institutes of Health to Y.L. (GM068496, GM062591) and N.L.G. (GM081597), the Welch Foundation (I-1560) to Y.L., the Cancer Prevention Research Institute of Texas (RP101496) to Z. X., and the Biotechnology and Biological Sciences Research Council (BBS/S/C2005/13012) to S.R.A. and S.K.C.
Author information
Authors and Affiliations
Contributions
Z.X., S.R.A., S.K.C. and Y.L. designed experiments. Z.X., S.R.A., J.Y. and D.K. performed experiments. Q.Y. provided technical support. Y.L., Z.X., S.R.A., N.L.G. and S.K.C. analysed data. Y.L., Z.X. and S.K.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
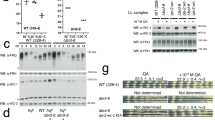
Extended Data Figure 1 Light-induced qrf expression represses frq transcription and regulates light resetting of the clock.
a, Strand-specific RNA-Seq result of the frq locus, showing the overlapping frq and qrf transcripts. b, Sequence alignment of known LRE elements. The qLRE and the mutated regions in the qrf promoter are shown. c, Diagrams showing the chromosomal modifications in the indicated loci in the frq10;frqWT and frq10;frqqLREmut strains. C, ClaI; B, BglII; E, EcoRV. d, Strand-specific RT–qPCR results showing the expression levels of frq and qrf in indicated strains in LL. Error bars show standard deviations (n = 3). **P < 0.01; n.s. indicates that the difference is not statistically significant. frq-RT and qrf -RT represent the non-RT reaction control. e, Diagram describing the strategy used to obtain the knock-in strains by homologous recombination. f, Strand-specific RT–qPCR results showing the expression levels of frq and qrf in the indicated knock-in strains in LL. g, Western blot results showing the FRQ expression levels in the indicated strains in LL. The densitometric analysis of western blot results from three independent experiments is shown at the right. Error bars show standard deviations. **P < 0.01. h, Phase response curves of circadian conidiation rhythms of the indicated knock-in strains after 2 min of light pulse at different circadian time (CT) points.
Extended Data Figure 2 qrf expression is required for circadian rhythmicities.
a, Strand-specific RT–qPCR results showing the expression levels of qrf in the indicated knock-in strains at DD24. b, Race tube analyses of the frq10;frqWT and frq10;frq.aq strains in medium containing 0% glucose and 0.17% arginine with the indicated concentrations of QA in DD. The lack of glucose in medium is known to allow more efficient expression from the qa-2 promoter. The black lines on race tubes indicate the daily growth fronts. c, The unnormalized luciferase activity of the experiments in Fig. 2c. d, The normalized result of Fig. 2c on a ×10 scale, showing that fluctuation of the luciferase activity in the frq10;frq.aq strain is random and not rhythmic. e, The phases of the first conidiation band in DD of the race tube results shown in Fig. 2b. f, Western blot analysis showing the FRQ expression profiles in the frq10;frq.aq strain in the presence and absence of QA in DD at the indicated time points. g, Northern blot analysis showing frq expression profiles in the frq10;frq.aq strain in DD at the indicated time points. The densitometric analysis is shown below.
Extended Data Figure 3 Regulation of qrf expression.
a, WC complex does not bind to the qrf promoter in DD. WC-2 ChIP assays showing the relative levels of WC binding at the frq (dLRE) and qrf (qLRE) promoters at the indicated time points in DD. b, Strand-specific RT–qPCR results showing the levels of frq and qrf at DD24 in the indicated strains. c, The unnormalized luciferase activity of the experiments in Fig. 3b. d, The normalized luciferase activity of the Pqrf-luc construct in Fig. 3b shown on a ×10 scale. e, Densitometric analyses of three independent northern blot results, indicating that levels of qrf transcripts are decreased in the frq9 strains as a result of increased frq expression.
Extended Data Figure 4 Rhythmic transcription of frq drives rhythmic qrf expression and mathematical modelling of the Neurospora circadian oscillator.
a, The unnormalized luciferase activity of the experiments in Fig. 3e. b, The unnormalized luciferase activity of the wild-type strain carrying the Pmin-luc construct. Results of two independent transformants are shown. c, Mathematical modelling of the Neurospora circadian oscillator with the double negative feedback loop. The differential equations used in the model are shown. The model is identical to a previously developed model23 with the exception of equation (1), which in this case includes the inhibition of frq transcription by qrf, and equation (8), which includes the inhibition of qrf transcription by frq. The rate constants used in the simulations are listed below.
Extended Data Figure 5 Neither RNAi nor DNA methylation pathways have a significant role in the clock.
a, b, Strand-specific RT–qPCR results showing the induction of frq after a light pulse are similar in the indicated strains at DD24. b, Strand-specific RT–qPCR results showing the induction of frq after a light pulse is similar in the indicated strains at DD24. c, Strand-specific RT–qPCR results showing similar expression levels of frq and qrf in the indicated strains in LL. d, A table showing the period lengths of the conidiation rhythms in the wild type and in different RNAi mutants. e, Strand-specific RT–qPCR results showing that the induction of frq after a light pulse is similar in the indicated strains at DD24. f, Strand-specific RT–qPCR results showing that the induction of frq after a light pulse is similar in the indicated strains at DD24. Error bars show standard deviations (n = 3).
Extended Data Figure 6 Characterization of the qrf action on frq expression.
a, Diagram showing the chromosomal modifications in the frqqLREmut;qrf strains that allow the expression of qrf in trans. The frqqLREmut construct is at the his-3 locus, and qrf is expressed only from the csr-1 locus. The red dashed line indicates that the frq promoter region is deleted in the qrf construct to abolish frq expression. b, Strand-specific RT–qPCR results showing that only qrf is expressed from the qrf construct in the frq10;qrf strain. c, qrf expression does not repress frq transcription in trans. Strand-specific RT–qPCR results showing the levels of frq and qrf transcripts in the indicated strains in LL. Error bars show standard deviations. *P < 0.05 (n = 3). d, Northern blot results showing that expression of qrf in trans in the frq10;frqqLREmut;qrf strains does not repress frq expression. Densitometric analysis of the northern blot results is shown at the right. e, WC-2 ChIP assays showing the relative WC binding levels at the frq promoter in LL in the indicated strains. The wc double mutant (wccDKO) was used as a negative control for ChIP. n.s. indicates a lack of statistical significance (n = 3). f, WC-2 ChIP assays showing the relative WC-2 binding levels at the frq promoter in LL in the indicated strains. The wc double mutant (wccDKO) was used as a negative control for the ChIP assays. n.s. indicates a lack of statistical significance (n = 3). g, Strand-specific RT–qPCR results showing that the mutation of the qLRE element in the qrf promoter results in significant increases in light-induced frq pre-mRNA expression. Nascent nuclear RNA was used. h, Northern blot results showing that the stability of frq mRNA is not affected by the transcription of qrf. The frq10;frq.aq strain that can induce qrf expression in the presence of QA was used. Thiolutin, a transcription inhibitor30, was added in the culture to block frq transcription so that frq mRNA stability could be determined. Cultures were harvested at the indicated time points after the addition of thiolutin.
Extended Data Figure 7 Modified Pol II CTD distribution profiles of a locus lacking antisense transcripts.
a, Top panel: strand-specific RNA-Seq results of the NCU01953 locus. Bottom panel: the positions of primers used in ChIP assays and the position of the riboprobe used in northern blot analysis. b, ChIP assays showing the relative enrichment of Pol II Ser 5 phosphorylation, Pol II Ser2 phosphorylation, and H3K36me3 at the NCU01953 locus in the wild-type strain. The scale on the y axis is the enrichment percentage of immunoprecipitation (IP) over input. IgG was used as the mock control for IP.
Extended Data Figure 8 Modified Pol II CTD distribution profiles at the frq locus.
a, Diagram showing the positions of primers at the frq locus used for ChIP assays and the positions of riboprobes used for northern blot analyses. b, ChIP assays showing the relative enrichment of histone H3 and Pol II CTD in the frq locus. The scale on the y axis is the enrichment percentage of immunoprecipitation (IP) over input. IgG was used as the mock control for IP. c, ChIP assays showing the relative enrichment of Pol II Ser 5 phosphorylation and Pol II Ser 2 phosphorylation (ChIP data in Fig. 4b were normalized by Pol II CTD ChIP results in b), and H3K36me3 (ChIP data in Fig. 4b were normalized by histone H3 ChIP results in b). *P < 0.05, **P < 0.01, ***P < 0.001 (n = 3).
Extended Data Figure 9 Complete abolition of qrf expression changes the distributions of modified pol II CTD to a normal profile.
a, Diagram showing the positions of primers used for ChIP assays. b, ChIP assays showing the relative enrichment of Pol II Ser 5 phosphorylation, Pol II Ser 2 phosphorylation, and H3K36me3 in the frq locus in the frq10;frq.aq strain in medium with 2% glucose. Under these conditions, qrf expression is completely abolished (shown in Fig. 2a). c, ChIP assays showing the relative enrichment of Pol II Ser 5 phosphorylation and Pol II Ser 2 phosphorylation (ChIP data in b were normalized by Pol II CTD ChIP results), and H3K36me3 (ChIP data in b were normalized by histone H3 ChIP results).
Extended Data Figure 10 Mechanism of qrf action on frq expression.
a, Densitometric analyses of three independent experiments shown in Fig. 4c. b, Densitometric analyses of independent experiments shown in Fig. 4d. c, Northern blot analysis using a N-term probe (marked in Extended Data Fig. 8a) specific for the 5′ half of qrf transcripts in the WT and WT;dsrrp44 strains in LL. d, Northern blot analysis using a N-term probe (marked in Extended Data Fig. 7a) specific for the 5′ half of NCU01953 transcripts in the WT;dsrrp44 strain in LL.
Rights and permissions
About this article
Cite this article
Xue, Z., Ye, Q., Anson, S. et al. Transcriptional interference by antisense RNA is required for circadian clock function. Nature 514, 650–653 (2014). https://doi.org/10.1038/nature13671
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13671
This article is cited by
-
A crucial role for dynamic expression of components encoding the negative arm of the circadian clock
Nature Communications (2023)
-
New insights into genome annotation in Podospora anserina through re-exploiting multiple RNA-seq data
BMC Genomics (2022)
-
CCIVR facilitates comprehensive identification of cis-natural antisense transcripts with their structural characteristics and expression profiles
Scientific Reports (2022)
-
Chromatin accessibility profiling in Neurospora crassa reveals molecular features associated with accessible and inaccessible chromatin
BMC Genomics (2021)
-
FRQ-CK1 interaction determines the period of circadian rhythms in Neurospora
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.