Abstract
Replying to F. Fieni et al. Nature 513, 10.1038/nature13626 (2014)
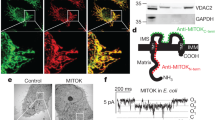
In our Letter1 identifying mitochondrial CaMKII as a crucial component of a Ca2+-dependent process of heart disease, we used multiple methods to show that CaMKII modulates mitochondrial Ca2+ homeostasis, as outlined below. First, we carried out electrophysiology of the mitochondrial calcium uniporter (MCU) current in mitoplasts. In our report1 we did not claim to measure capacitance of the mitoplast separately from the total capacitance of the mitoplast and pipette. Although we concede that the approach of Fieni et al.2 is preferable, we found that even after removing any correction for capacitance, dialysis with constitutively active CaMKII monomers increased MCU current whereas dialysis with catalytically dead CaMKII monomers did not.
Similar content being viewed by others
Main
Second, we identified candidate CaMKII sites on the MCU amino terminus (serine 57 and serine 92), and used a variety of approaches to provide evidence that both are functional and their phosphorylation by CaMKII increases MCU current. These approaches include transgenic mitochondrial-targeted expression of a highly selective CaMKII inhibitor (mtCaMKIIN), and dialysis of both constitutively active and catalytically inactive control CaMKII in cardiac mitoplasts, as well as in mitoplasts from HEK cells overexpressing wild-type MCU or a mutant form in which the two serines are replaced with alanines (S57A/S92A). We are uncertain why the dialysis by Fieni et al.2, which used a constitutively active monomeric form of CaMKII, was ineffective in increasing MCU current, but given the tendency with which this kinase may lose activity we prefer a positive control experiment showing that the introduced CaMKII is active within a biological system.
Third, we measured a current from mitoplasts that we believe to be from MCU because: (1) it rectifies inwardly; (2) it displays greater Na+ than Ca2+ conductance; and (3) it is inhibited by Ru360 (albeit with a different kinetic signature than reported by Fieni et al.2). In addition to the voltage-clamp approach, we measured mitochondrial Ca2+ entry with Ca2+ green-5N dye and mitochondrial-targeted cameleon FRET, which also report on mitochondrial Ca2+ entry via the MCU pathway (that is, MCU current), as confirmed by a recent report on MCU knockout mice3. Indeed, mitochondrial Ca2+ uptake measured using Ca2+ green-5N, intramitochondrial Ca2+ sensitive probes and Ca2+-induced mitochondrial swelling are absent in MCU knockout mice, indicating that these approaches are reporting on MCU current. These approaches, deployed in a variety of systems, corroborated our findings with voltage clamp and supported the role of CaMKII in facilitating mitochondrial Ca2+ entry.
Since the time of our publication we have continued to study the role of CaMKII in mitochondria, including work to validate the Ser 57 and Ser 92 sites on MCU further. We note that our ongoing experiments support the concepts outlined in our original paper. The main finding in Joiner et al. was that CaMKII is present in cardiac mitochondria, where it participates in mitochondrial Ca2+ homeostasis and contributes to pathological responses to stress, including myocardial infarction, ischaemia-reperfusion injury and catecholamine toxicity1. We stand by the findings in our report, and look forward to future studies that will shed light on the contribution of MCU, its regulatory subunits and signalling pathways to mitochondrial biology and disease.
References
Joiner, M. A. et al. CaMKII determines mitochondrial stress responses in heart. Nature 491, 269–273 (2012)
Fieni, F., Johnson, D. E., Hudmon, A. & Kirichok, Y. Mitochondrial Ca2+ uniporter and CaMKII in heart. Nature 513 http://dx.doi.org/10.1038/nature13626 (2014)
Pan, X. et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nature Cell Biol. 15, 1464–1472 (2013)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Joiner, Ml., Koval, O., Li, J. et al. Joiner et al. reply. Nature 513, E3 (2014). https://doi.org/10.1038/nature13627
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13627
This article is cited by
-
Mitochondrial CaMKII causes adverse metabolic reprogramming and dilated cardiomyopathy
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.