Abstract
Haematopoietic stem cells (HSCs) self-renew for life, thereby making them one of the few blood cells that truly age1,2. Paradoxically, although HSCs numerically expand with age, their functional activity declines over time, resulting in degraded blood production and impaired engraftment following transplantation2. While many drivers of HSC ageing have been proposed2,3,4,5, the reason why HSC function degrades with age remains unknown. Here we show that cycling old HSCs in mice have heightened levels of replication stress associated with cell cycle defects and chromosome gaps or breaks, which are due to decreased expression of mini-chromosome maintenance (MCM) helicase components and altered dynamics of DNA replication forks. Nonetheless, old HSCs survive replication unless confronted with a strong replication challenge, such as transplantation. Moreover, once old HSCs re-establish quiescence, residual replication stress on ribosomal DNA (rDNA) genes leads to the formation of nucleolar-associated γH2AX signals, which persist owing to ineffective H2AX dephosphorylation by mislocalized PP4c phosphatase rather than ongoing DNA damage. Persistent nucleolar γH2AX also acts as a histone modification marking the transcriptional silencing of rDNA genes and decreased ribosome biogenesis in quiescent old HSCs. Our results identify replication stress as a potent driver of functional decline in old HSCs, and highlight the MCM DNA helicase as a potential molecular target for rejuvenation therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013)
Rossi, D. J., Jamieson, C. H. & Weissman, I. L. Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008)
Rossi, D. J. et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199 (2005)
Chambers, S. M. et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 5, e201 (2007)
Geiger, H., de Haan, G. & Florian, M. C. The ageing haematopoietic stem cell compartment. Nature Rev. Immunol. 13, 376–389 (2013)
Rossi, D. J. et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–729 (2007)
Rübe, C. E. et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS ONE 6, e17487 (2011)
Ciccia, A. & Elledge, S. J. The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204 (2010)
Nijnik, A. et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 447, 686–690 (2007)
Welch, J. S. et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 150, 264–278 (2012)
Mohrin, M. et al. Hematopoietic stem cell quiescence promotes error prone DNA repair and mutagenesis. Cell Stem Cell 7, 174–185 (2010)
Burhans, W. C. & Weinberger, M. DNA replication stress, genome instability and aging. Nucleic Acids Res. 35, 7545–7556 (2007)
Pietras, E. M., Warr, M. R. & Passegué, E. Cell cycle regulation in hematopoietic stem cells. J. Cell Biol. 195, 709–720 (2011)
Branzei, D. & Foiani, M. Maintaining genome stability at the replication fork. Nature Rev. Mol. Cell Biol. 11, 208–219 (2010)
Lukas, C. et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nature Cell Biol. 13, 243–253 (2011)
Méndez, J. & Stillman, B. Chromatin association of human origin recognition complex, Cdc6 and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20, 8602–8612 (2000)
Aparicio, T., Guillou, E., Coloma, J., Montoya, G. & Méndez, J. The human GINS complex associates with Cdc45 and MCM and is essential for DNA replication. Nucleic Acids Res. 37, 2087–2095 (2009)
Ibarra, A., Schwob, E. & Méndez, J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc. Natl Acad. Sci. USA 105, 8956–8961 (2008)
Zhong, Y. et al. The level of origin firing inversely affects the rate of replication fork progression. J. Cell Biol. 201, 373–383 (2013)
Pruitt, S. C., Bailey, K. J. & Freeland, A. Reduced Mcm2 expression results in severe stem/progenitor cell deficiency and cancer. Stem Cells 25, 3121–3132 (2007)
Lanctôt, C., Cheutin, T., Cremer, M., Cavalli, G. & Cremer, T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nature Rev. Genet. 8, 104–115 (2007)
Boisvert, F. M., van Koningsbruggen, S., Navascués, J. & Lamond, A. I. The multifunctional nucleolus. Nature Rev. Mol. Cell Biol. 8, 574–585 (2007)
Durkin, S. G. & Glover, T. W. Chromosome fragile sites. Annu. Rev. Genet. 41, 169–192 (2007)
Nakada, S., Chen, G. I., Gingas, A.-C. & Durocher, D. PP4 is a γH2AX phosphatase required for recovery from the DNA damage checkpoint. EMBO Rep. 9, 1019–1026 (2008)
Fumagalli, M. et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nature Cell Biol. 14, 355–365 (2012)
Beerman, I., Seita, J., Inlay, M. A., Weissman, I. L. & Rossi, D. J. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell 15, 37–50 (2014)
Rando, T. A. & Chang, H. Y. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell 148, 46–57 (2012)
Narla, A., Hurst, S. N. & Ebert, B. L. Ribosome defects in disorders of erythropoiesis. Int. J. Hematol. 93, 144–149 (2011)
Warr, M. R. et al. FoxO3a directs a protective autophagy program in hematopoietic stem cells. Nature 494, 323–327 (2013)
Cooley, C. et al. Trf1 is not required for proliferation or functional telomere maintenance in chicken DT40 cells. Mol. Biol. Cell 20, 2563–2571 (2009)
Terret, M. E., Sherwood, R., Rahman, S., Qin, J. & Jallepalli, P. V. Cohesin acetylation speeds the replication fork. Nature 462, 231–234 (2009)
Smyth, G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article 3 (2004)
Norddahl, G. L. et al. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell 8, 499–510 (2011)
Bersenev, A. et al. Lnk deficiency partially mitigates hematopoietic stem cell aging. Aging Cell 11, 949–959 (2012)
Will, B. et al. Satb1 regulates the self-renewal of hematopoietic stem cells by promoting quiescence and repressing differentiation commitment. Nature Immunol. 14, 437–445 (2013)
Olive, P. L. & Banath, J. P. The comet assay: a method to measure DNA damage in individual cells. Nature Protocols 1, 23–29 (2006)
Acknowledgements
We thank A. Brunet and S. Villeda for providing some old C57BL/6 mice, B. McStay for advice on nucleolar analyses, C. Klijn for assistance with microarray analyses, S. Katzman for the SNP analyses, E. Davis for help with cytogenetic studies, I. Grummt for the rDNA plasmid, M. Kissner and M. Lee for management of our Flow Cytometry Core Facility, and all members of the Passegué laboratory for critical insights and suggestions. S.T.B. and M.M. were supported by a California Institute for Regenerative Medicine (CIRM) training grant and E.M.P. by National Institutes of Health (NIH) F32 HL106989. This work was supported by Science Foundation Ireland PI award 10/IN.1/B2972 to C.G.M. and a CIRM New Faculty Award RN2-00934 and NIH R01 HL092471 to E.P.
Author information
Authors and Affiliations
Contributions
J.F. performed all of the experiments with help from S.T.B. for the comet assays and microarray data analyses, E.M.P. for the Ki67/DAPI staining and microarray analyses, and D.R. for the transplantation experiments. M.M. and P.C.C. initiated these studies. S.A. and J.M. performed the DNA replication track analyses and helped with the MCM experiments, M.E.D. and B.A.S. performed the telomere analyses, F.U. and E.C.F. performed the SNP analyses, and M.M.L.B. performed the cytogenetic breakage studies and spectral karyotyping analyses. J.F., C.G.M. and E.P. designed the experiments and interpreted the results. J.F. and E.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
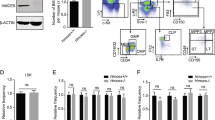
Extended Data Figure 1 Isolation strategy and functional impairment of old HSCs.
a, Gating strategy used to isolate HSCs (Lin−/Sca1+/c-Kit+/Flk2−/CD150+/CD48−), MPPs (Lin−/Sca1+/c-Kit+/Flk2+) and GMPs (Lin−/Sca1−/c-Kit+/FcγR+/CD34+) from the bone marrow of young (6–12 weeks) and old (22–30 months) C57BL/6 mice. o, old; y, young. b, Reconstitution ability of young and old HSCs. HSCs were isolated from C57BL/6-CD45.2 donor mice, and transplanted (250 HSCs per mouse) into lethally irradiated young C57BL/6-CD45.1 recipients (n = 5 mice per cell type) together with 300,000 Sca1-depleted CD45.1 helper bone marrow cells. The percentage of donor-derived chimaerism and myeloid (light blue) versus lymphoid (dark blue) reconstitution in the peripheral blood was assessed by flow cytometry at the indicated months post-transplantation. Stars indicate phenotypes resembling age-related blood disorders: black, bone marrow failure; white, myeloproliferative neoplasm.
Extended Data Figure 2 Age-associated γH2AX signals are not associated with markers of DNA damage.
a, Additional images for γH2AX staining in young and old HSCs. b, Additional images for DNA damage markers in old HSCs. c, Quantification of DNA damage marker staining shown in Fig. 1c, d. Results are expressed as per cent of positive cells. d, Representative images of young and old HSCs analysed by alkaline comet assay showing a field view of all cells and examples of normal (<3 MAD) and outliers (≥3 MAD) cells. MAD, median absolute deviation in mean tail moment. e, Mean tail moment for young and old HSCs in one representative alkaline comet experiment. Results are expressed as boxplots with the line marking the median, the box the boundaries of the 25th and 75th percentiles, and the whiskers the 90th and 10th percentiles. f, Mean tail moment for young and old HSCs compared with 0.5 Gy irradiated young HSCs analysed by alkaline comet assay. Results are expressed as boxplots as in e. ***P ≤ 0.001 (pairwise Mann–Whitney rank sum test). g, Representative images of γH2AX/53BP1 foci in 0.5 Gy irradiated young HSCs. Scale bars, 10 µm (a, b, g); 90 µm (magnified cells, 60 µm) (d).
Extended Data Figure 3 Signal intensity, repair kinetics and residual replication stress in old HSCs.
a, Representative images comparing γH2AX signal intensity in unirradiated young and old HSCs, and 2 Gy irradiated young HSCs. Two different intensity settings for the 488 nm laser are used. b, Additional images for the clearance of γH2AX/53BP1 foci in 2 Gy irradiated young and old HSCs. c, Additional images for γH2AX/RPA and γH2AX/ATRIP staining in young and old HSCs. Scale bars, 10 µm.
Extended Data Figure 4 Consequences of replication stress in cycling old HSCs.
a, Representative FACS plots for Ki67/DAPI intracellular staining in young and old HSCs. Two independent examples of old HSC staining are shown. b, qRT–PCR analyses of cell cycle gene expression in young and old HSCs (n = 5). Results are expressed as fold change compared with young HSCs (set to 1). Data are means ± s.d. **P ≤ 0.01. c, Representative images of 17 h single EdU (insert) and 21 h double EdU/BrdU labelling experiments. d, Schematic and representative images of γH2AX and γH2AX/EdU staining in cycling young and old HSCs re-isolated 2 weeks after transplantation. e, Additional images of persistent 53BP1 bodies in 36 h cycling young and old HSCs that have re-entered G1 following replication. G1-phase cells were identified as cells with mean DAPI intensity × area ≤ 11,000, and represented ∼49% (young HSCs) and ∼52% (old HSCs) of the population at that time. f, Representative field images and mean tail moment for 36 h cycling young and old HSCs analysed by alkaline comet assay. Results are expressed as boxplots with the line marking the median, the box the boundaries of the 25th and 75th percentiles, and the whiskers the 90th and 10th percentiles. Two independent examples of comet assays are shown. g, Mean tail moment for day 2 cyclophosphamide (C)/G-CSF mobilized young and old HSCs analysed by alkaline comet assay. Results are expressed as boxplots as in f. *P ≤ 0.05, ***P ≤ 0.001 (pairwise Mann–Whitney rank sum test). Scale bars, 100 µm (c); 10 µm (d, e); 90 µm (f).
Extended Data Figure 5 Rare cases of exacerbated replication stress and identification of MCM defect in old HSCs.
a, Representative images of senescence-associated β-galactosidase (SA-β-Gal) staining and qRT–PCR analyses of Cdkn2a (p16) expression levels in young and old HSCs. Results are expressed as log2 fold changes compared with young HSCs (set to 0). Only 2 out of 10 preparations of old HSCs showed SA-β-Gal staining, while only 2 out of 9 preparations of young HSCs and 4 out of 12 preparations of old HSCs had detectable p16 levels. Of note, old HSCs with the highest p16 levels also scored positive for SA-β-Gal staining. b, Representative images of telomere FISH on metaphase spreads of young and old HSCs. Magnified inserts show detection of multiple telomeric signals (asterisks) in old HSCs, and histograms indicate the per cent of all telomeres with multiple telomeric signals per young and old HSCs (7 and 6 cells scored, respectively). Only 2 out of 6 preparations of old HSCs showed multiple telomeric signals. Data are means ± s.d. c, d, Microarray analysis showing differential Mcm gene expression in young and old HSCs (c) and GMPs (d). A total of 5 (young) and 3 (old) independent biological replicates were used for HSCs, and 4 (young) and 3 (old) for GMPs. Results are expressed as boxplots with the line marking the median, the box the boundaries of the 25th and 75th percentiles and the whiskers the ±1.5 interquartile range. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 (two-sided t-test). e, qRT–PCR analyses of Mcm gene expression in young and old GMPs (n = 3–5). Results are expressed as fold change compared with young GMPs (set to 1). Data are means ± s.d. Scale bars, 100 µm; insert, 10 µm (a); 10 µm; magnified cells, 1 µm (b).
Extended Data Figure 6 Specific decrease in Mcm gene expression in old HSCs.
a, b, qRT–PCR analyses of the expression of Mcm genes and other components of the pre-replication complex in quiescent (a) and cycling (b) young and old HSCs (n = 4–5). Results are expressed as fold change compared with young HSCs (set to 1). Data are means ± s.d. *P ≤ 0.05, **P ≤ 0.01. c, Re-analyses of published data sets for Mcm gene expression in old HSCs. Data from GEO accession number GSE27686 (ref. 33): HSCs were defined as Lin−/c-Kit+/Sca1+/CD34−/CD150+ with 3 young (10–12 weeks) and 3 old (100 weeks) samples. Data from GEO accession GSE39553 (ref. 34): HSCs were defined as Lin−/c-Kit+/Sca1+/CD48−/CD150+ with 3 young (2 months) and 4 old (20 months) samples. Data from GEO accession number GSE6503 (ref. 4): HSCs were defined as Lin−/c-Kit+/Sca1+/Hoechst-3342low with 2 young (2 months) and 2 old (21 months) samples. Data from GEO accession number GSE4332 (ref. 3): HSCs were defined as Lin−/c-Kit+/Sca1+/CD34−/Flk2− with 3 young (2–3 months) and 5 old (22–24 months) samples. Results are heatmaps of expression measurements for probes mapping to Mcm genes with non-supervised hierarchical clustering using Euclidean distance and Ward’s linkage.
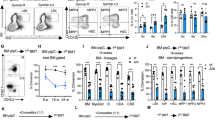
Extended Data Figure 7 Consequences of replication stress and decreased Mcm expression for HSC function.
a, Effect of replication stress on the reconstitution ability of young and old HSCs. HSCs were isolated from C57BL/6-CD45.2 donor mice, treated with aphidicolin (Aph; 50 ng ml−1) or vehicle (Veh; DMSO) for 36 h in vitro and transplanted (250 HSCs per mouse) into lethally irradiated young C57BL/6-CD45.1 recipients (n = 5 mice per cell type) together with 300,000 Sca1-depleted CD45.1 helper bone marrow cells. The percentage of donor-derived chimaerism and myeloid (light blue) versus lymphoid (dark blue) reconstitution in the peripheral blood was assessed by flow cytometry at the indicated months post-transplantation. Black star indicates bone marrow failure, dagger indicates animal mortality. b, Donor-derived chimaerism in the HSC compartment of the surviving mice at 4 months post-transplantation (n = 3–5). c, Differential killing of young and old HSCs after 72 h treatment. Eto, etoposide (0.25 µM); HU, hydroxyurea (100 µM); Stau, staurosporine (5 nM). Results are normalized for vehicle-treated cells and expressed as fold change compared with young HSCs (set to 1). d, Additional images of CldU/IdU-labelled stretched DNA fibres from replicating young and old HSCs. e, f, Effect of lentiviral-mediated knockdown of Mcm4 and Mcm6 on young HSCs (n = 3): e, additional images for MCM4 and MCM6 protein levels (with MFI quantification) and γH2AX foci; f, qRT–PCR analyses of Mcm4 and Mcm6 expression levels. Transduced GFP+ HSCs were re-isolated 48 h post-infection. Results are expressed as fold change compared with scrambled shRNA (scr)-infected HSCs. Two independent shRNA constructs are used per gene. UT, untransfected. Data are means ± s.d. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. NS, not significant. Scale bars, 1.5 µm insert, 3.5 µm (d); 10 µm (e).
Extended Data Figure 8 Replication stress leads to persistence of nucleolar-associated γH2AX signals in quiescent old HSCs.
a, Functional effect of lentiviral knockdown of Mcm4 and Mcm6 on young HSCs, and direct comparison with old HSCs in liquid culture expansion (left) and methylcellulose (right). Transduced GFP+ HSCs were re-isolated 48 h post-infection. Results are expressed as fold change compared with scrambled shRNA (scr)-infected HSCs for transduced HSCs, and young HSCs for old HSCs. Two independent shRNA constructs are used per gene. b, Representative images of γH2AX and centromere marker (CENP-A) staining and immuno-FISH for γH2AX and telomeric PNA probe in young and old HSCs. c, Additional images for γH2AX and nucleolar marker co-localization in old HSCs. d, Representative images of γH2AX and nucleolar marker co-localization in old MPPs, GMPs and granulocytes (Gr). e, Percentage of young and old cells with nucleolar staining (HSC: 445 and 464; MPP: 485 and 390; GMP: 179 and 223 cells scored, respectively) and representative electron microscopy images of young and old HSCs and GMPs. Data are means ± s.d. White asterisks indicate nucleoli. f, Representative images of γH2AX/FBL staining in cultured young and old HSCs. g, Additional images of immuno-FISH for γH2AX and rDNA probe in young and old HSCs. Scale bars, 10 µm (b, c, d, f, g); 2 µm (e).
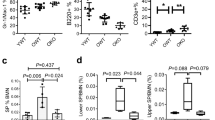
Extended Data Figure 9 Decreased ribosome biogenesis and mis-localization of the PP4c phosphatase in quiescent old HSCs.
a, Schematic and representative images of γH2AX/FBL staining in cycling and quiescent donor-derived young and old HSCs re-isolated at the indicated times after transplantation. b, RNA Bioanalyzer track showing the predominant 5.8S, 18S and 28S rRNA peaks, and quantification of RNA content in quiescent young and old HSCs (n = 4). Results are expressed as area under the curve divided by the total number of cells in each sample. Data are means ± s.d. *P ≤ 0.05. c, Representative images of NPM1 (nucleolar marker) and the indicated histone methylation mark in young and old HSCs. d, Additional images of PP4c staining in cycling young and old HSCs. Scale bars, 10 µm.
Extended Data Figure 10 Model for replication stress and γH2AX accumulation in old HSCs.
In contrast to HSCs isolated from young mice (yHSC), which replicate normally, HSCs isolated from old mice (oHSC) have severe replication defects due to decreased expression of mini-chromosome maintenance (MCM) DNA helicase components. Cycling old HSCs show heightened levels of replication-associated γH2AX foci (small green stars) and increased levels of replication stress associated with cell cycle defects, altered DNA fork replication dynamics and chromosome gaps/breaks. Nonetheless, old HSCs usually survive replication unless confronted with a strong replication challenge such as treatment with a replication stressor drug or transplantation, which preferentially kill them and lead to their defective engraftment in vivo. Cycling old HSCs are also likely to accumulate γH2AX foci on rDNA genes as a consequence of replication stress occurring at these hard-to-replicate loci during their replication in late S phase. However, stalled or collapsed replication forks appear to be repaired upon activation of the canonical DNA damage response (DDR), resulting in essentially undamaged rDNA genes in old HSCs. In contrast, the clearance of γH2AX foci is probably unfinished by the time old HSCs re-enter quiescence, thereby aggregating leftover γH2AX signals in reformed nucleoli in post-mitotic cells. We propose that ineffective de-phosphorylation of γH2AX due to mislocalization of PP4c phosphatase in quiescent old HSCs contributes to the long-term persistence of nucleolar-associated γH2AX signals (large green stars) in the absence of ongoing DNA damage and DDR activation. Persistent nucleolar γH2AX also acts in a non-canonical manner as a histone modification marking the transcriptional silencing of rDNA genes and decreased ribosome biogenesis in quiescent old HSCs. Whether decreased rDNA transcription in quiescent old HSCs is involved in bone marrow (BM) failure syndromes associated with defective ribosome biogenesis (ribosomopathy) remains to be determined. However, as soon as old HSCs re-enter the cell cycle, PP4c is re-localized to the nucleus and quickly dephosphorylates nucleolar γH2AX, thereby restoring ribosome biogenesis. Our results demonstrate that accumulation of γH2AX in old HSCs marks either ongoing or residual replication stress caused by the inability of ageing HSCs to maintain normal levels of MCM proteins. They highlight the MCM DNA helicase as a potential molecular target for rejuvenation therapies.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-2. (PDF 173 kb)
Rights and permissions
About this article
Cite this article
Flach, J., Bakker, S., Mohrin, M. et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 512, 198–202 (2014). https://doi.org/10.1038/nature13619
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13619
This article is cited by
-
Implications of stress-induced gene expression for hematopoietic stem cell aging studies
Nature Aging (2024)
-
Aging-induced MCPH1 translocation activates necroptosis and impairs hematopoietic stem cell function
Nature Aging (2024)
-
Deregulated protein homeostasis constrains fetal hematopoietic stem cell pool expansion in Fanconi anemia
Nature Communications (2024)
-
Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity
Nature (2024)
-
Fanca deficiency is associated with alterations in osteoclastogenesis that are rescued by TNFα
Cell & Bioscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.