Abstract
β-Thalassaemia major (β-TM) is an inherited haemoglobinopathy caused by a quantitative defect in the synthesis of β-globin chains of haemoglobin, leading to the accumulation of free α-globin chains that form toxic aggregates1,2. Despite extensive knowledge of the molecular defects causing β-TM, little is known of the mechanisms responsible for the ineffective erythropoiesis observed in the condition, which is characterized by accelerated erythroid differentiation, maturation arrest and apoptosis at the polychromatophilic stage3,4,5,6. We have previously demonstrated that normal human erythroid maturation requires a transient activation of caspase-3 at the later stages of maturation7. Although erythroid transcription factor GATA-1, the master transcriptional factor of erythropoiesis, is a caspase-3 target, it is not cleaved during erythroid differentiation. We have shown that, in human erythroblasts, the chaperone heat shock protein70 (HSP70) is constitutively expressed and, at later stages of maturation, translocates into the nucleus and protects GATA-1 from caspase-3 cleavage8. The primary role of this ubiquitous chaperone is to participate in the refolding of proteins denatured by cytoplasmic stress, thus preventing their aggregation9. Here we show in vitro that during the maturation of human β-TM erythroblasts, HSP70 interacts directly with free α-globin chains. As a consequence, HSP70 is sequestrated in the cytoplasm and GATA-1 is no longer protected, resulting in end-stage maturation arrest and apoptosis. Transduction of a nuclear-targeted HSP70 mutant or a caspase-3-uncleavable GATA-1 mutant restores terminal maturation of β-TM erythroblasts, which may provide a rationale for new targeted therapies of β-TM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Khandros, E. & Weiss, M. J. Protein quality control during erythropoiesis and hemoglobin synthesis. Hematol. Oncol. Clin. North Am. 24, 1071–1088 (2010)
Ginzburg, Y. & Rivella, S. β-thalassemia: a model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood 118, 4321–4330 (2011)
Yuan, J. et al. Accelerated programmed cell death (apoptosis) in erythroid precursors of patients with severe β-thalassemia (Cooley’s anemia). Blood 82, 374–377 (1993)
Mathias, L. A. et al. Ineffective erythropoiesis in β-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp. Hematol. 28, 1343–1353 (2000)
Centis, F. et al. The importance of erythroid expansion in determining the extent of apoptosis in erythroid precursors in patients with β-thalassemia major. Blood 96, 3624–3629 (2000)
Ribeil, J. A. et al. Ineffective erythropoiesis in β-thalassemia. ScientificWorldJournal 2013, 394295 (2013)
Zermati, Y. et al. Caspase activation is required for terminal erythroid differentiation. J. Exp. Med. 193, 247–254 (2001)
Ribeil, J.-A. et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature 445, 102–105 (2007)
Hartl, F. U., Bracher, A. & Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 (2011)
Hu, J. et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood 121, 3246–3253 (2013)
Kihm, A. J. et al. An abundant erythroid protein that stabilizes free α-haemoglobin. Nature 417, 758–763 (2002)
Frisan, E. et al. Defective nuclear localization of Hsp70 is associated with dyserythropoiesis and GATA-1 cleavage in myelodysplastic syndromes. Blood 119, 1532–1542 (2012)
De Maria, R. et al. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature 401, 489–493 (1999)
Dover, G. J. & Boyer, S. H. Fetal hemoglobin-containing cells have the same mean corpuscular hemoglobin as cells without fetal hemoglobin: a reciprocal relationship between gamma- and beta-globin gene expression in normal subjects and in those with high fetal hemoglobin production. Blood 69, 1109–1113 (1987)
Yao, X. et al. Role of STAT3 and GATA-1 interactions in gamma-globin gene expression. Exp. Hematol. 37, 889–900 (2009)
Woon Kim, Y., Kim, S., Geun Kim, C. & Kim, A. The distinctive roles of erythroid specific activator GATA-1 and NF-E2 in transcription of the human fetal γ-globin genes. Nucleic Acids Res. 39, 6944–6955 (2011)
Zhu, J. et al. Recombinant erythroid Kruppel-like factor fused to GATA1 up-regulates δ- and γ-globin expression in erythroid cells. Blood 117, 3045–3052 (2011)
Sankaran, V. G. & Orkin, S. H. The switch from fetal to adult hemoglobin. Cold Spring Harb. Perspect. Med. 3, a011643 (2013)
Libani, I. V. et al. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in beta-thalassemia. Blood 112, 875–885 (2008)
Ramos, P. et al. Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia. Nature Med. 19, 437–445 (2013)
Dussiot, M. et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia. Nature Med. 20, 398–407 (2014)
Suragani, R. N. V. S. et al. Modified activin receptor IIB ligand trap mitigates ineffective erythropoiesis and disease complications in murine β-thalassemia. Blood http://dx.doi.org/10.1182/blood-2013-06-511238 (19 June 2014)
De Franceschi, L. et al. K-CL co-transport plays an important role in normal and beta thalassemic erythropoiesis. Haematologica 92, 1319–1326 (2007)
Zermati, Y. et al. Transforming growth factor inhibits erythropoiesis by blocking proliferation and accelerating differentiation of erythroid progenitors. Exp. Hematol. 28, 885–894 (2000)
Gabet, A.-S. et al. Caspase-activated ROCK-1 allows erythroblast terminal maturation independently of cytokine-induced Rho signaling. Cell Death Differ. 18, 678–689 (2011)
Bolte, S. & Cordelières, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006)
Schallmeiner, E. et al. Sensitive protein detection via triple-binder proximity ligation assays. Nature Methods 4, 135–137 (2007)
Kiger, L. et al. Dynamics of α-Hb chain binding to its chaperone AHSP depends on heme coordination and redox state. Biochim. Biophys. Acta 1840, 277–287 (2014)
Abraham, E. C., Reese, A., Stallings, M. & Huisman, T. H. Separation of human hemoglobins by DEAE-cellulose chromatography using glycine-KCN-NaC1 developers. Hemoglobin 1, 27–44 (1976)
Bucci, E. & Fronticelli, C. A new method for the preparation of alpha and beta subunits of human hemoglobin. J. Biol. Chem. 240, 551–552 (1965)
Anson, M. L. & Mirsky, A. E. Protein coagulation andits reversal: the preparation of insoluble globin, soluble globin and heme. J. Gen. Physiol. 13, 469–476 (1930)
Acknowledgements
J.-B.A. was a recipient of grants from the Société Nationale Française de Médecine Interne (SNFMI), Société Française d’Hématologie (SFH), Centre National de la Recherche Scientifique (CNRS) and Assistance publique –Hôpitaux de Paris (AP–HP) (poste d’accueil APHP-CNRS), and from Groupe Pasteur Mutualité. S.D. was a recipient of funding from Institut FARMAN (ENS de Cachan). I.C.B. was a recipient of a PhD scholarship fund from ENS de Cachan. A.H. was a recipient of a post-doctoral grant from the Conseil Régional de Bourgogne and M.S. from l’Institut National du Cancer, INCa. This program has received a state subsidy managed by the National Research Agency under the ‘Investments for the Future’ program bearing the reference ANR-10-IAHU-01, Ligue nationale contre le cancer, Fondation pour la recherché médicale (FRM) and Fondation de France. The labex GR-Ex is funded by the program ‘Investments for the Future’ of the French National Research Agency, reference ANR-11-IDEX-0005-02. We would also like to thank N. Goudin and R. Desvaux from the Plateforme d'imagerie cellulaire de l'IFR94, Faculté de Médecine Necker (Paris, France) for experimental help in image acquisition and analysis on confocal microscopy, and the Department of Biotherapy at the Necker Hospital (Paris, France) for providing blood samples. We thank P. England from CNRS 1129, unité de biochimiecellulaire, Institut Pasteur, Paris, for his experimental help. We thank E. Frisan from the Department of Haematology and INSERM U1016, Hôpital Cochin, Paris, and A. de Thonel from INSERM U866, Dijon, for the gift of HSP70(S400A) mutant. We thank U. Testa, A. Zeuner and R. de Maria from the Department of Haematology and Oncology, Instituto Superiore di Santia, Rome, Italy, for the gift of cDNAs of GATA-1. We also thank C. Brouzes from the Department of biological haematology for her help for the smears’ reading.
Author information
Authors and Affiliations
Contributions
J.-B.A. designed and performed all experiments, analysed the data and wrote the manuscript. J.-A.R. designed the study, supervised the overall project, provided human samples, analysed the data and helped to write the manuscript. A.H., M.S. and C.G. performed the yeast two-hybrid assays and analysed the data. G.M. and R.S. performed the biolayer interferometry (Octet) and helped to write the manuscript. C.G., S.D., I.C.B., L.T., C.A., V.B.-C.and Y.B. performed experiments to characterise the HSP70/α-globin complex and helped to write the manuscript. F.G. performed biochemical experiments and helped to write the manuscript. M.D., I.C.M., Z.B.-C., M.F. and T.T.M. analysed the data and helped to write the manuscript. O.N., P.L., S.C. and Y.B. performed the β-globin gene lentiviral transduction and HPLC analyses. O.H. designed the study, supervised the overall project, analysed the data and wrote the manuscript. G.C. performed experiments, supervised the overall project, analysed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
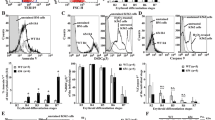
Extended Data Figure 1 Kinetics of differentiation of β-TM and control erythroblasts and cell-cycle analysis.
CD36+ cells derived from CD34+ adult β-TM or healthy (control) peripheral blood cells were cultured, as described in Methods. Differentiation staining was assessed at several points during CD36+ cell culture by flow cytometry. a, Differentiation curves determined using (top panel) band-3 MFI (n = 4 independent experiments), (middle panel) glycophorin-A (GpA) MFI and (bottom panel) CD117 MFI. For GpA and CD117, n = 19 independent experiments in β-TM group and n = 11 in control group. P values compare erythroid differentiation between β-TM and control cell cultures at day 8. b, Representative flow cytometry plots (GpA and CD117 staining) showing difference in erythroid differentiation at day 8. GpA+/CD117− cells represent mature erythroblasts. In the bottom panel, histograms of band-3 staining in β-TM and peripheral blood stem cells (PBSC, control erythroblasts) at day 8 are shown (Ctl-Ab, isotypic control antibody). c, Cell cycle was analysed at day 4 and day 8 of CD36+ cell cultures from β-TM and healthy donors (n = 4 independent experiments). Representative flow cytometry histograms at day 8 are shown in the bottom panel. Data in panels a and c are presented as mean ± s.e.m. P values determined by two-tailed Student’s unpaired t-tests. *P < 0.05, ***P < 0.001.
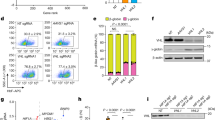
Extended Data Figure 2 Specific developmental stage purification of erythroblasts and biochemical analysis of HSP70 and GATA-1 nuclear content.
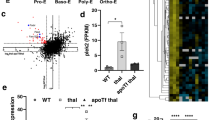
a, Strategy for flow cytometry (FACS) purification of erythroblasts at specific developmental stages at day 8 from CD36+β-TM or control-derived cell cultures. Band-3 and α4-integrin staining were used to isolate three stages of erythroid differentiation as described in Methods (representative β-TM-derived cell culture is shown). Numbers indicate the percentage of cells from each gate. Scale bar, 10 µm. b, A representative morphological analysis (×100) of cells purified from each gate by MGG staining. Gate I, basophil erythroblasts (Baso), gate II, polychromatophilic erythroblasts (Poly), gate III, acidophilic erythroblasts. Scale bar, 10 µm. c, HSP70 and GATA-1 or α-globin western blot from nuclear (upper panel) or cytoplasmic (lower panel) fractions extracted from FACS-purified gate I and II β-TM or control-derived cell cultures (day 8). HDAC2 and Hsp90 were used as controls for the nuclear and cytoplasmic fractions, respectively. Graphs show optical density values of specific protein normalized to that of HDAC2 or Hsp90. Data are representative of three independent experiments.
Extended Data Figure 3 HSP70–α-globin complexes correlate with the degree of cell differentiation.
Protein interactions were analysed at day 8 from CD36+β-TM or control-derived cell cultures by Duolink in situ proximity ligation assay, using HSP70 and α-globin antibodies. The red spots indicate close proximity between cellular-bound antibodies. More spots per cell are exhibited in more differentiated β-TM erythroblasts (smaller cells). A representative experiment is shown (n = 3). Nuclei are stained with DAPI (blue). Scale bar, 5 µm.
Extended Data Figure 4 Comparative study of apo-α-globin association to HSP70 or to AHSP by biolayer interferometry.
Sensorgrams are shown in blue and the corresponding fits in red. The data produced a Kd of 4.65 nM and 8.35 nM for HSP70–apo-α-globin (a) and AHSP–apo-α-globin (b) interactions, respectively. χ2was below 1.3 and R2was above 0.99 for all included sensorgrams. Data are representative of two independent experiments.
Extended Data Figure 5 Kinetics of cytoplasmic HSP70 and AHSP expression during erythropoiesis.
CD36+ cells derived from CD34+ adult β-TM or healthy (control) peripheral blood cells were cultured, as described in Methods section. a, b, HSP70, AHSP and α-globin expression was assessed in cytoplasm at several culture times (days 4, 6 and 8 of the CD36+ cell culture) by western blot analysis. a, A representative western blot is shown (n = 3 independent experiments). β-TM- and control-cell lysates have been loaded in the same gel. b, Graphs show the optical density values of specific proteins normalized to cytoplasmic protein HSC70.
Extended Data Figure 6 Absolute number of very mature erythroblasts increases with nuclear HSP70 and GATA-1 restoration.
Comparison of the absolute number of very mature erythroblasts (acidophilic cells + reticulocytes) at day 7 of CD36+ cell culture after transduction of β-TM CD34+ cells with a nuclear-targeted HSP70 lentiviral mutant (HSP70(S400A)) or a retroviral mutant of GATA-1 uncleavable by activated caspase-3 (µGATA-1) and their empty vector as control. Data are presented as mean ± s.e.m. for three independent experiments for each transduction. P values were determined using Student’s unpaired t-test. **P < 0.01.
Extended Data Figure 7 The lentiviral transduction of β-TM CD34+ cells with the β-globin gene rescues HSP70 nuclear localization and GATA-1 protection.
β-TM CD34+ cells derived from peripheral blood cells were infected with a lentivirus encoding the β-globin gene or a control lentivirus (n = 2, 10–15% transduction efficiency). They were then cultured with a two-phase amplification liquid culture system, as described in Methods. Results at day 8 of CD36+ cell culture are shown. a, Confocal microscopy analysis of HSP70 nuclear localization and GATA-1 expression for each transduction. Scale bar, 10 µm. b, c, Erythroid differentiation. The graph in b represents the proportion of cells at each developmental stage after MGG staining for each transduction (mean ± s.e.m.). P values were determined by two-tailed Student’s unpaired t-tests. **P < 0.01; ns, not significant. Representative flow cytometry plots of band-3 and α4-integrin staining are shown in c, left panel. The terminal maturation index of this experiment as calculated by flow cytometry is shown in c, right panel.
Rights and permissions
About this article
Cite this article
Arlet, JB., Ribeil, JA., Guillem, F. et al. HSP70 sequestration by free α-globin promotes ineffective erythropoiesis in β-thalassaemia. Nature 514, 242–246 (2014). https://doi.org/10.1038/nature13614
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13614
This article is cited by
-
Gene expression changes in sickle cell reticulocytes and their clinical associations
Scientific Reports (2023)
-
Role of Caspase-10-P13tBID axis in erythropoiesis regulation
Cell Death & Differentiation (2023)
-
Cabozantinib promotes erythroid differentiation in K562 erythroleukemia cells through global changes in gene expression and JNK activation
Cancer Gene Therapy (2022)
-
Lactobacillus stress protein GroEL prevents colonic inflammation
Journal of Gastroenterology (2021)
-
Oxidative Stress in β-Thalassemia
Molecular Diagnosis & Therapy (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.