Abstract
Necroptosis has emerged as an important pathway of programmed cell death in embryonic development, tissue homeostasis, immunity and inflammation1,2,3,4,5,6,7,8. RIPK1 is implicated in inflammatory and cell death signalling9,10,11,12,13 and its kinase activity is believed to drive RIPK3-mediated necroptosis14,15. Here we show that kinase-independent scaffolding RIPK1 functions regulate homeostasis and prevent inflammation in barrier tissues by inhibiting epithelial cell apoptosis and necroptosis. Intestinal epithelial cell (IEC)-specific RIPK1 knockout caused IEC apoptosis, villus atrophy, loss of goblet and Paneth cells and premature death in mice. This pathology developed independently of the microbiota and of MyD88 signalling but was partly rescued by TNFR1 (also known as TNFRSF1A) deficiency. Epithelial FADD ablation inhibited IEC apoptosis and prevented the premature death of mice with IEC-specific RIPK1 knockout. However, mice lacking both RIPK1 and FADD in IECs displayed RIPK3-dependent IEC necroptosis, Paneth cell loss and focal erosive inflammatory lesions in the colon. Moreover, a RIPK1 kinase inactive knock-in delayed but did not prevent inflammation caused by FADD deficiency in IECs or keratinocytes, showing that RIPK3-dependent necroptosis of FADD-deficient epithelial cells only partly requires RIPK1 kinase activity. Epidermis-specific RIPK1 knockout triggered keratinocyte apoptosis and necroptosis and caused severe skin inflammation that was prevented by RIPK3 but not FADD deficiency. These findings revealed that RIPK1 inhibits RIPK3-mediated necroptosis in keratinocytes in vivo and identified necroptosis as a more potent trigger of inflammation compared with apoptosis. Therefore, RIPK1 is a master regulator of epithelial cell survival, homeostasis and inflammation in the intestine and the skin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Oberst, A. et al. Catalytic activity of the caspase-8–FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011)
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011)
Zhang, H. et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471, 373–376 (2011)
Bonnet, M. C. et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35, 572–582 (2011)
Welz, P. S. et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330–334 (2011)
Dillon, C. P. et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 1, 401–407 (2012)
Duprez, L. et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35, 908–918 (2011)
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 (2010)
Kelliher, M. A. et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 (1998)
Cusson-Hermance, N., Khurana, S., Lee, T. H., Fitzgerald, K. A. & Kelliher, M. A. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-κB activation but does not contribute to interferon regulatory factor 3 activation. J. Biol. Chem. 280, 36560–36566 (2005)
Meylan, E. et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-κB activation. Nature Immunol. 5, 503–507 (2004)
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nature Immunol. 6, 981–988 (2005)
Rajput, A. et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity 34, 340–351 (2011)
Christofferson, D. E., Li, Y. & Yuan, J. Control of life-or-death decisions by RIP1 kinase. Annu. Rev. Physiol. 76, 129–150 (2014)
Vanden Berghe, T., Linkermann, A., Jouan-Lanhouet, S., Walczak, H. & Vandenabeele, P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nature Rev. Mol. Cell Biol. 15, 135–147 (2014)
Hill, D. A. & Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 28, 623–667 (2010)
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010)
Lee, T. H., Shank, J., Cusson, N. & Kelliher, M. A. The kinase activity of Rip1 is not required for tumor necrosis factor-α-induced IκB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J. Biol. Chem. 279, 33185–33191 (2004)
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004)
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007)
Steinbrecher, K. A., Harmel-Laws, E., Sitcheran, R. & Baldwin, A. S. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J. Immunol. 180, 2588–2599 (2008)
Sasaki, Y. et al. Canonical NF-κB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity 24, 729–739 (2006)
Vlantis, K. et al. Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J. Clin. Invest. 121, 2781–2793 (2011)
Gentle, I. E. et al. In TNF-stimulated cells, RIPK1 promotes cell survival by stabilizing TRAF2 and cIAP1, which limits induction of non-canonical NF-κB and activation of caspase-8. J. Biol. Chem. 286, 13282–13291 (2011)
Kim, J. Y. et al. TNFα induced noncanonical NF-κB activation is attenuated by RIP1 through stabilization of TRAF2. J. Cell Sci. 124, 647–656 (2011)
Piao, J. H. et al. Tumor necrosis factor receptor-associated factor (TRAF) 2 controls homeostasis of the colon to prevent spontaneous development of murine inflammatory bowel disease. J. Biol. Chem. 286, 17879–17888 (2011)
Varfolomeev, E. et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131, 669–681 (2007)
Vince, J. E. et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 (2007)
Polykratis, A. et al. Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. http://dx.doi.org/10.4049/jimmunol.1400590 (2014)
Mc Guire, C. et al. Oligodendrocyte-specific FADD deletion protects mice from autoimmune-mediated demyelination. J. Immunol. 185, 7646–7653 (2010)
Rodríguez, C. I. et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nature Genet. 25, 139–140 (2000)
Schwenk, F., Baron, U. & Rajewsky, K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23, 5080–5081 (1995)
Madison, B. B. et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 (2002)
El Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004)
Hafner, M. et al. Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis 38, 176–181 (2004)
Pfeffer, K. et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73, 457–467 (1993)
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998)
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004)
Fu, Y., Sander, J. D., Reyon, D., Cascio, V. M. & Joung, J. K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nature Biotechnol. 32, 279–284 (2014)
Ukena, S. N. et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE 2, e1308 (2007)
Schmidt-Supprian, M. et al. NEMO/IKKγ-deficient mice model incontinentia pigmenti. Mol. Cell 5, 981–992 (2000)
Kumari, S. et al. Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity 39, 899–911 (2013)
Klose, C. S. et al. A T-bet gradient controls the fate and function of CCR6−RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013)
Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013)
Snippert, H. J., Schepers, A. G., Delconte, G., Siersema, P. D. & Clevers, H. Slide preparation for single-cell-resolution imaging of fluorescent proteins in their three-dimensional near-native environment. Nature Protocols 6, 1221–1228 (2011)
Tscharntke, M. et al. Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J. Cell Sci. 120, 1480–1490 (2007)
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009)
Acknowledgements
We are grateful to V. Dixit for Ripk3−/−, D. Gumucio for Villin-Cre and S. Robine for Villin-CreERT2 mice. We thank C. Uthoff-Hachenberg, J. Buchholz, E. Mahlberg, B. Kühnel, B. Hülser, P. Jankowski, S. Assenmacher and P. Scholl for technical assistance. M.P. acknowledges funding from the European Research Council (2012-ADG_20120314), the German Research Council (DFG; SFB670, SFB829, SPP1656), the European Commission (grants 223404 (Masterswitch) and 223151 (InflaCare)), the Deutsche Krebshilfe, the Else Kröner-Fresenius-Stiftung and the Helmholtz Alliance (PCCC). Research reported in this publication was also supported by the National Institute of Allergy and Infectious Diseases division of the National Institutes of Health under award RO1AI075118 to M.K.
Author information
Authors and Affiliations
Contributions
M.D. together with K.V. performed and analysed the experiments related to the intestine and S.K. performed and analysed the experiments related to the skin. N.H. and M.K. designed and generated the targeting constructs for the Ripk1fl/fl and Ripk1D138N/D138N mice. A.P. performed the gene targeting in embryonic stem cells and generated the Ripk1fl/fl, Ripk1D138N/D138N and Triffl/fl mice. M.Z. contributed to the analysis of intestines from Ripk1−/− neonates. C.K. and J.L. performed biochemical analysis of RIPK1-deficient MEFs, IECs and keratinocytes. C.E. performed FACS analysis of intestinal immune cells. T.C. performed qRT–PCR analysis. L.W. designed and tested the short guiding RNAs for CRISPR/Cas9-mediated targeting of Mlkl. P.K., M.B. and A.B. generated germ-free RIPK1IEC-KO mice. M.P. coordinated the project and together with K.V., M.D. and S.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
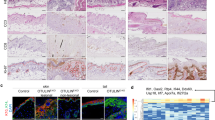
Extended Data Figure 1 Intestinal pathology in Ripk1−/− and RIPK1IEC-KO mice.
a, Representative images of colonic and ileal sections from newborn Ripk1+/+ and Ripk1−/− mice stained with H&E or immunostained for CC3. b, Targeting strategy for the generation of mice with loxP-flanked (floxed (fl)) Ripk1 alleles. Exon 3 of the Ripk1 gene was flanked with loxP sites and an FRT-flanked neomycin (Neo) selectable cassette was introduced after the 3′ loxP site. The Neo cassette was excised by crossing the Ripk1neoFloxed mice with Flp-deleter mice. c, Representative Southern blots depicting the identification of correctly targeted embryonic stem (ES) cell clones by using 5′ and 3′ external probes. Arrows indicate a correctly targeted ES cell clone. Screening for the loxP site in intron 2 was performed by PCR (data not shown). Double combs allowing loading samples at two levels were used to maximize loading capacity of the gels for screening ES clones in 96-well plates. d, Immunoblot of colon IEC protein extracts from Ripk1fl/fl and RIPK1IEC-KO mice. e, f, Representative images of ileal (e) or colon (f) sections from Ripk1fl/fl and RIPK1IEC-KO mice stained with H&E, PAS or alkaline phosphatase (AP), or immunostained against lysozyme, CC3 or Ki67. Scale bars, 100 µm.
Extended Data Figure 2 Mild intestinal inflammation in RIPK1IEC-KO mice.
a, qRT–PCR analysis of cytokine and chemokine expression in colon tissue from Ripk1fl/fl and RIPK1IEC-KO mice. b–d, FACS analysis of lamina propria leukocytes in the small intestine (b) and colon (c, d) of Ripk1fl/fl and RIPK1IEC-KO mice. e, Representative images from intestinal sections from Ripk1fl/fl and RIPK1IEC-KO mice immunostained with the indicated antibodies. Scale bars, 100 µm. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005; NS, not significant.
Extended Data Figure 3 Assessment of intestinal pathology in newborn and 1-week-old RIPK1IEC-KO mice.
a, Representative images of intestinal sections from Ripk1fl/fl and RIPK1IEC-KO mice stained with H&E or immunostained with the indicated antibodies. Arrows indicate sparse CC3+ IECs in sections from 1-day-old animals. Scale bars, 100 µm. b, Quantification of crypts containing CC3+ cells and the number of CC3+ cells per crypt in intestinal sections of 1-week-old Ripk1fl/fl and RIPK1IEC-KO mice. c, d, qRT–PCR analysis of cytokine and chemokine expression in small intestinal and colon tissue from 1-day-old (c) and 1-week-old (d) Ripk1fl/fl and RIPK1IEC-KO mice. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005; NS, not significant.
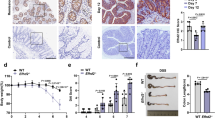
Extended Data Figure 4 Antibiotic treatment does not prevent intestinal pathology in RIPK1IEC-KO and RIPK1tamIEC-KO mice.
a, Experimental outline of tamoxifen injections (TAM; 1 mg, intraperitoneally). b, Immunoblot of small intestine IEC protein extracts from tamoxifen-treated Ripk1fl/fl and RIPK1tamIEC-KO mice. c–e, Body weight change (c), Kaplan–Meier survival curve (d) and representative images of H&E- and CC3-stained intestinal sections (e) of tamoxifen-treated Ripk1fl/fl and RIPK1tamIEC-KO mice receiving antibiotics (+AB) or normal drinking water starting 4 weeks before tamoxifen administration. f, g, Body weight change (f) and representative images of H&E- and CC3-stained intestinal sections (g) in vehicle-injected Ripk1fl/fl and RIPK1tamIEC-KO mice. h, i, Body weight changes (h) and representative images of H&E-stained intestinal sections (i) of tamoxifen-injected Villin-CreERT2 mice (n = 3). j–l, Body weight (j), Kaplan–Meier survival curve (k) and representative images of H&E- and CC3-stained intestinal sections (l) of Ripk1fl/fl and RIPK1IEC-KO mice treated with antibiotics from E17.5 to 3 weeks of age. Scale bars, 100 µm. Error bars represent mean values ± s.d. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005; NS, not significant.
Extended Data Figure 5 Role of MyD88, TNFR1, FADD and RIPK3 in the intestinal pathology of RIPK1IEC-KO mice.
a, Representative images of ileal sections from the indicated mice as controls for the mice shown in Fig. 2a. b, Quantification of histological pathology score of the indicated mice. c, Representative images of H&E- or CC3-stained colon sections from the indicated mice. d, Representative images of H&E- or CC3-stained ileal sections from the indicated mice. Scale bars, 100 µm. e, Quantification of histological pathology score of the indicated mice. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005; NS, not significant.
Extended Data Figure 6 Epithelial-specific FADD deficiency reduces IEC apoptosis, ameliorates crypt atrophy but triggers erosive lesions in the colon of RIPK1IEC-KO mice.
Representative images of ileal and colonic sections from the indicated mice stained with H&E or immunostained against lysozyme or CC3. Scale bars, 100 µm.
Extended Data Figure 7 FADD and RIPK3 deficiency restores intestinal homeostasis in RIPK1IEC-KO mice and IEC necroptosis in FADDIEC-KO mice occurs in the absence of RIPK1 kinase activity.
a, Representative images of H&E-stained colonic and ileal sections of adult mice with the indicated genotypes. b, Representative images of colonic and ileal sections of mice with the indicated genotypes immunostained for CD45. c, d, Representative images of colonic (c) and ileal (d) sections of adult Faddfl/fl/Ripk1D138N/D138N and FADDIEC-KO/Ripk1D138N/D138N mice stained with H&E or immunostained against CC3. Scale bars, 100 µm.
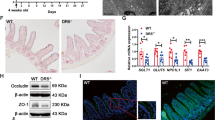
Extended Data Figure 8 Assessment of the role of NF-κB and TRIF signalling in RIPK1IEC-KO mice.
a, Immunoblot analysis of protein extracts from wild-type (WT), Ripk1−/− and Ripk1D138N/D138N MEFs after stimulation with 10 ng ml−1 recombinant murine TNF for the indicated time points. Data are representative of five independent experiments. b–d, Body weight (b), quantification of histological pathology score (c) and representative H&E-, CC3- or CD45-stained intestinal sections (d) of mice with the indicated genotypes. e, Targeting strategy used for the generation of Triffl/fl mice. Exon 2 of the Trif gene was flanked with loxP sites. FRT-flanked neo and a stop cassette were placed upstream of the 3′ loxP site. A splice acceptor (SA)-mCherry cassette was introduced downstream of the 3′ loxP site. Cre-mediated recombination removes the Trif coding sequences and the stop cassette inducing the expression of mCherry by the Trif locus. The FRT-flanked neo was excised by crossing TrifneoFloxed mice with Flp-Deleter mice. f, Confocal microscopy images of near native small intestinal sections of Triffl/fl and TRIFIEC-KO mice stained with anti-lysozyme and DAPI. g, h, Body weight (g) and representative images of H&E-, CC3- or CD45-stained intestinal sections (h) of the indicated mice. Scale bars, 100 µm. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005; NS, not significant.
Extended Data Figure 9 Skin inflammation in RIPK1E-KO mice is dependent on RIPK3-mediated necroptosis.
a, Representative macroscopic images of RIPK1E-KO mice. b, qRT–PCR analysis of pro-inflammatory cytokines and chemokines on total skin mRNA from Ripk1fl/fl and RIPK1E-KO mice. c, d, Representative images of skin sections from the indicated mice stained as indicated. In d, arrows point to CC3+ cells and arrowheads depict CC3− dying cells identified by their pyknotic nuclei and eosinophilic cytoplasm. Scale bars, 100 µm in H&E stained; 50 µm in immunostained sections. e–g, Representative macroscopic pictures (e, f) and H&E-stained skin sections (g) of the indicated mice. Scale bars, 100 µm. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005; NS, not significant.
Extended Data Figure 10 CRISPR/Cas9-mediated knockout of MLKL prevents skin inflammation in RIPK1E-KO mice.
a, Schematic depiction of the Mlkl locus. The sequence targeted by the small guide RNAs (sgRNA and truncated TRUNC_sgRNA) is indicated by capital letters. The position of the ATG and the binding sites of the primers used for genotyping and sequencing are indicated. The sgRNAs were designed to target a sequence containing an ApaLI restriction site that is used for RFLP analysis (underlined). The protospacer-adjacent motif (PAM) sequence is depicted in red. b, Sequences of the wild-type (WT) Mlkl locus and of the targeted Mlkl alleles of the two obtained RIPK1E-KO/Mlkl−/− mice. Mouse #150 carries one allele with one base pair (bp) deletion and one allele with one bp insertion. Mouse #374 carries one allele with one bp insertion and one allele with two bp insertions. All mutations cause frameshift and ablate MLKL protein expression. Mouse #150 was obtained using the full-length MLKL-sgRNA and mouse #374 was obtained using the truncated MLKL_sgRNA_TRUNC. c, Macroscopic appearance and histological images of skin sections from RIPK1E-KO/Mlkl−/− mouse #150 at the age of P95. Scale bars, 100 μm (H&E); 50 μm (keratins and CC3).
Rights and permissions
About this article
Cite this article
Dannappel, M., Vlantis, K., Kumari, S. et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513, 90–94 (2014). https://doi.org/10.1038/nature13608
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13608
This article is cited by
-
Mediators of necroptosis: from cell death to metabolic regulation
EMBO Molecular Medicine (2024)
-
Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity
Nature Reviews Cancer (2024)
-
Epithelial CRL4DCAF2 Is Critical for Maintaining Intestinal Homeostasis Against DSS-Induced Colitis by Regulating the Proliferation and Repair of Intestinal Epithelial Cells
Digestive Diseases and Sciences (2024)
-
PRMT5-mediated regulatory arginine methylation of RIPK3
Cell Death Discovery (2023)
-
Housing conditions affect enterocyte death mode and turnover rate in mouse small intestine
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.