Abstract
FOXP3+ regulatory T cells (Treg cells) are abundant in the intestine, where they prevent dysregulated inflammatory responses to self and environmental stimuli. It is now appreciated that Treg cells acquire tissue-specific adaptations that facilitate their survival and function1; however, key host factors controlling the Treg response in the intestine are poorly understood. The interleukin (IL)-1 family member IL-33 is constitutively expressed in epithelial cells at barrier sites2, where it functions as an endogenous danger signal, or alarmin, in response to tissue damage3. Recent studies in humans have described high levels of IL-33 in inflamed lesions of inflammatory bowel disease patients4,5,6,7, suggesting a role for this cytokine in disease pathogenesis. In the intestine, both protective and pathological roles for IL-33 have been described in murine models of acute colitis8,9,10,11, but its contribution to chronic inflammation remains ill defined. Here we show in mice that the IL-33 receptor ST2 is preferentially expressed on colonic Treg cells, where it promotes Treg function and adaptation to the inflammatory environment. IL-33 signalling in T cells stimulates Treg responses in several ways. First, it enhances transforming growth factor (TGF)-β1-mediated differentiation of Treg cells and, second, it provides a necessary signal for Treg-cell accumulation and maintenance in inflamed tissues. Strikingly, IL-23, a key pro-inflammatory cytokine in the pathogenesis of inflammatory bowel disease, restrained Treg responses through inhibition of IL-33 responsiveness. These results demonstrate a hitherto unrecognized link between an endogenous mediator of tissue damage and a major anti-inflammatory pathway, and suggest that the balance between IL-33 and IL-23 may be a key controller of intestinal immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Burzyn, D., Benoist, C. & Mathis, D. Regulatory T cells in nonlymphoid tissues. Nature Immunol. 14, 1007–1013 (2013)
Pichery, M. et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33–LacZ gene trap reporter strain. J. Immunol. 188, 3488–3495 (2012)
Palmer, G. & Gabay, C. Interleukin-33 biology with potential insights into human diseases. Nature Rev. Rheumatol. 7, 321–329 (2011)
Beltrán, C. J. et al. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 16, 1097–1107 (2010)
Kobori, A. et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J. Gastroenterol. 45, 999–1007 (2010)
Pastorelli, L. et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc. Natl Acad. Sci. USA 107, 8017–8022 (2010)
Seidelin, J. B. et al. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol. Lett. 128, 80–85 (2010)
Oboki, K. et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl Acad. Sci. USA 107, 18581–18586 (2010)
Duan, L. et al. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3+ regulatory T-cell responses in mice. Mol. Med. 18, 753–761 (2012)
Groβ, P., Doser, K., Falk, W., Obermeier, F. & Hofmann, C. IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm. Bowel Dis. 18, 1900–1909 (2012)
Sedhom, M. A. et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut 62, 1714–1723 (2013)
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005)
Thornton, A. M. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441 (2010)
Wang, Y., Su, M. A. & Wan, Y. Y. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 35, 337–348 (2011)
Wohlfert, E. A. et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J. Clin. Invest. 121, 4503–4515 (2011)
Rudra, D. et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nature Immunol. 13, 1010–1019 (2012)
Guo, L. et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl Acad. Sci. USA 106, 13463–13468 (2009)
Maneechotesuwan, K. et al. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J. Immunol. 178, 2491–2498 (2007)
Furusawa, J. et al. Critical role of p38 and GATA3 in natural helper cell function. J. Immunol. 191, 1818–1826 (2013)
Turnquist, H. R. et al. IL-33 expands suppressive CD11b+ Gr-1int and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J. Immunol. 187, 4598–4610 (2011)
Wasserman, A. et al. Interleukin-33 augments Treg cell levels: a flaw mechanism in atherosclerosis. Isr. Med. Assoc. J. 14, 620–623 (2012)
Kullberg, M. C. et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 203, 2485–2494 (2006)
Hayakawa, H., Hayakawa, M., Kume, A. & Tominaga, S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J. Biol. Chem. 282, 26369–26380 (2007)
Díaz-Jiménez, D. et al. Soluble ST2: a new and promising activity marker in ulcerative colitis. World J. Gastroenterol. 17, 2181–2190 (2011)
Zheng, Y. et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812 (2010)
Wan, Y. Y. & Flavell, R. A. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 445, 766–770 (2007)
Kitoh, A. et al. Indispensable role of the Runx1-Cbfβ transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity 31, 609–620 (2009)
Maloy, K. J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 (2011)
Izcue, A. et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 28, 559–570 (2008)
Ahern, P. P. et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33, 279–288 (2010)
Fontenot, J. D. et al. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 22, 329–341 (2005)
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006)
Read, S., Malmstrom, V. & Powrie, F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192, 295–302 (2000)
Uhlig, H. H. et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 177, 5852–5860 (2006)
Hue, S. et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203, 2473–2483 (2006)
Krausgruber, T. et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nature Immunol. 12, 231–238 (2011)
Acknowledgements
C.S., K.A., A.C., O.J.H. and F.P. are supported by the Wellcome Trust. F.P. is also supported by the Fondation Louis Jeantet. A.N.H. is supported by a European Molecular Biology Organization long-term fellowship (ALTF 116-2012). P.G.F. is supported by Science Foundation Ireland. M.L. and A.F. are supported by the Volkswagen Foundation (Lichtenberg Program) and BMBF (e:Bio/T-Sys). B.M.J.O. is supported by an Oxford-UCB Pharma Postdoctoral Fellowship. We thank all members of the Oxford Translational Gastroenterology Unit for assistance and support. We are grateful to H. Ferry and K. Alford for essential flow cytometry support and the staff of the University of Oxford for animal care. We are also grateful to D. Baban for conducting microarray hybridizations.
Author information
Authors and Affiliations
Contributions
C.S. and T.K. planned experiments and analysed the data. C.S., T.K. and F.P. wrote the paper. A.C., A.F., K.A., O.J.H., A.N.H., E.A.W., T.G., J.B., B.M.J.O. and J.P. performed particular experiments. M.L., Y.B. and P.G.F. provided essential materials and were involved in data discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Effects of IL-33 on gene expression during iTreg differentiation.
a, Sort-purified CD25−CD62L+CD44lo GFP− naive CD4+ T cells from Foxp3gfp reporter mice were stimulated with anti-CD3/anti-CD28 in the presence of indicated cytokines. mRNA expression of indicated genes was measured at 24 h, 48 h and 72 h and presented as fold change over time 0. Data are representative of two independent experiments and show the mean ± s.d.
Extended Data Figure 2 IL-33 directly regulates ST2 expression.
Sort-purified CD25−CD62L+CD44lo GFP− naive CD4+ T cells from Foxp3gfp reporter mice were cultured with anti-CD3/anti-CD28 in the presence of TGF-β1 followed by acute stimulation with IL-33 for 45 min. Shown are the recruitment of GATA3 or RNA Pol II to the indicated regions of the gene encoding ST2 assessed by ChIP followed by qPCR. Results are normalized to those obtained with genomic DNA (input) and presented as fold enrichment relative to unstimulated cells. Data are from one experiment representative of two (error bars show s.d.).
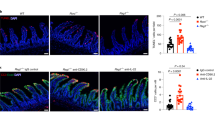
Extended Data Figure 3 IL-33 acts directly on thymus-derived Treg cells to promote their proliferation and accumulation in vivo.
a–e, Foxp3gfp reporter mice were injected intraperitoneally (i.p.) with recombinant IL-33 (1 µg per mouse per day) for 5 days. a, Frequencies of TCR-β+CD4+Foxp3+ splenic thymus-derived Treg cells. b, Absolute numbers of TCR-β+CD4+Foxp3+ splenic Treg cells. c, gMFI of Foxp3 among TCRβ+CD4+Foxp3+ splenic Treg cells (n = 10 for PBS and n = 14 for IL-33). d, Frequencies of ST2+ cells among splenic CD44hi T cells or Foxp3+ Treg cells. e, Frequencies of Ki67+ cells among splenic CD44hi T cells or Foxp3+ Treg cells (n = 5 for PBS and n = 8 for IL-33). f, Mixed bone marrow chimaeras were generated by irradiation of C57BL/6 Rag1−/− mice followed by intravenous (i.v.) injection of 2.5 × 106 wild-type (WT; CD45.1+) and 2.5 × 106 St2−/− (CD45.1−) bone marrow cells (n = 5). After reconstitution, mixed chimaeras were injected with recombinant IL-33 (1 µg per mouse per day) for 5 days and the absolute number of Ki67+ among TCR-β+CD4+Foxp3− or TCRβ+CD4+Foxp3+ cells are shown. **P < 0.01, ***P < 0.001 as calculated by unpaired (wild-type mice) or paired (chimaeric mice), Student’s t-test.
Extended Data Figure 4 Kinetics of IL-33 and IL-23 during H. hepaticus and anti-IL-10R colitis.
a–f, C57BL/6 mice were infected by oral gavage with ∼108 colony-forming units (c.f.u.) H. hepaticus on 3 consecutive days with concomitant i.p. injections of a blocking anti-IL-10R monoclonal antibody (1 mg per week) starting on the day of the first infection. a, b, Colitis scores for the indicated groups H. hepaticus (Hh), anti-IL-10R (aIL-10R) or H. hepaticus and anti-IL-10R (Hh/aIL-10R) (a) and IL-33, IL-23 and soluble ST2 protein production (b) in colon explant cultures from H. hepaticus and anti-IL-10R treated mice were determined at the indicated time points (n = 4, data show the mean ± s.e.m.). c, Colonic intestinal epithelial cells (IECs) were isolated from steady state (n = 5) or colitic (n = 3) (day 10) mice. Total protein extracts from each time point were analysed by immunoblot and biological replicates are shown. d, Densitometric quantification of immunoblot in c (mean ± s.e.m.). e, mRNA expression analysis of IECs, lamina propria cells (LP) and stromal cells isolated from the colon at steady state or 10 days after induction of colitis (steady state, n = 5; colitic, n = 4; mean ± s.e.m.). f, Colonic stromal cells were isolated at the indicated time points, cultured and passaged and spontaneous soluble (s)ST2 protein production determined in supernatants after 48 h cultures. Stromal cells were uniformly CD45−EpCAM−. Data represent mean ± s.e.m. (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 as calculated by one-way ANOVA with Bonferroni post-test or Student’s t-test.
Extended Data Figure 5 The in vitro suppressive function of St2−/ − Treg cells is not impaired.
CD25+CD4+ Treg cells were sorted by flow cytometry from wild-type (WT) and St2−/− mice. CD25−CD62L+CD44−CD4+ T cells (responder cells) and antigen presenting cells (APCs) were sorted from St2−/− mice. T responder cells were labelled with Violet cell trace and plated together with the sorted Treg cells at ratios of 1:1 and 1:3 together with irradiated APCs in the presence of anti-CD3 (1 μg ml−1). IL-33 was added at 30 ng ml−1. After 4 days, proliferation of T responder cells (Tresp) was measured by flow cytometry. Data are representative of two independent experiments.
Extended Data Figure 6 ST2 is preferentially expressed on Treg-cell progeny.
a, C57BL/6 Rag1−/− mice were injected i.p. with 4 × 105 CD4+CD25−CD45 RBhi CD45.1+ naive T cells in combination with 2 × 105 wild-type (WT) CD4+CD25+ CD45.1− Treg cells. Mice were killed at 6–8 weeks after transfer. Representative plots of the colon gated on progeny of CD4+CD45 RBhi cells (CD45.1+) or wild-type Treg (CD45.1−) cells are shown. b, Histogram overlay of Foxp3 expression by wild-type (solid line) or St2−/− (dotted line) CD4+CD25+ Treg cells before injection into C57BL/6 Rag1−/− mice. c, C57BL/6 Rag1−/− mice were injected as in a and frequencies of cytokine-producing colonic Treg progeny (CD45.1−) among Foxp3− or Foxp3+ cells are shown. Wild type (n = 4) and St2−/− (n = 6); error bars represent mean ± s.e.m.
Extended Data Figure 7 St2 is an IL-23 target gene in intestinal CD4+ T cells.
C57BL/6 Rag1−/− mice were injected i.p. with a 1:1 mixture of 2 × 105 wild-type (WT; CD45.1+) and 2 × 105 Il23r−/− (CD45.2+) CD4+CD25−CD45 RBhi T cells or 2 × 105 wild-type (CD45.1+) and 2 × 105 wild-type (CD45.2+) CD4+CD25−CD45 RBhi T cells. Mice were killed upon development of clinical signs of inflammation (∼8 weeks). Viable TCR-β+CD4+CD45.2+ wild-type or Il23r−/− T cells were sort-purified from the colon and RNA extracted without further manipulation. Whole transcriptome gene expression analysis was performed using the Illumina Bead Array platform. Two-dimensional cluster analysis (hierarchical, Pearson uncentred, average linkage, unsupervised) of the 168 transcripts whose expression was significantly affected in the comparison of Il23r−/− versus wild-type CD4+ T cells. Each row corresponds to a gene and each column to a sample (n = 6 for wild type and n = 5 for Il23r−/− ). The 168 transcripts represent 147 unique genes, including genes with unknown function.
Extended Data Figure 8 IL-23 inhibits IL-33-induced GATA3 recruitment to the St2 locus.
Sort-purified CD25−CD62L+CD44lo GFP− naive CD4+ T cells from Foxp3gfp reporter mice were cultured with anti-CD3/anti-CD28/TGF-β1 +/− IL-23 for 48 h followed by acute stimulation with IL-33 for 45 min. Shown are the enrichment of GATA3 or RNA Pol II to the indicated regions of the gene encoding ST2 assessed by ChIP followed by qPCR. Results are normalized to those obtained with genomic DNA (input) and presented as fold recruitment relative to unstimulated cells. -, no IL-33 added. Data are from one experiment representative of two (error bars show s.d. of duplicate cultures).
Extended Data Figure 9 IL-23 is a negative regulator of ST2 expression in vivo.
Mixed bone marrow chimaeras were generated by irradiation of C57BL/6 Rag1−/− mice followed by i.v. injection of 2.5 × 106 wild-type (WT; CD45.1+) and 2.5 × 106 Il23r−/− (CD45.1−) bone marrow cells (n = 5). Reconstituted mice were infected by oral gavage with ∼108 c.f.u. H. hepaticus on 3 consecutive days with concomitant i.p. injections of a blocking anti-IL-10R monoclonal antibody (1 mg per week) starting on the day of the first infection. Mice were killed at 2 weeks after infection. Frequencies of ST2+ and gMFI of ST2 among TCR-β+CD4+Foxp3+ colonic Treg cells are shown. *P < 0.05, ***P < 0.001 as calculated by paired Student’s t-test.
Extended Data Figure 10 Il23r mRNA is expressed by ST2+Foxp3+ Treg cells.
mRNA expression of indicated genes in Foxp3−ST2−, Foxp3+ST2− or Foxp3+ST2+ populations sort-purified from the steady state colonic lamina propria (cLP) of Foxp3gfp reporter mice. AU, arbitrary units. Error bars represent the mean ± s.e.m. from three independent experiments.
Rights and permissions
About this article
Cite this article
Schiering, C., Krausgruber, T., Chomka, A. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014). https://doi.org/10.1038/nature13577
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13577
This article is cited by
-
TMED10-mediated unconventional secretion of IL-33 regulates intestinal epithelium differentiation and homeostasis
Cell Research (2024)
-
A type 1 immunity-restricted promoter of the IL−33 receptor gene directs antiviral T-cell responses
Nature Immunology (2024)
-
CD4+ and CD8+ regulatory T cell characterization in the rat using a unique transgenic Foxp3-EGFP model
BMC Biology (2023)
-
Focal adhesion kinase: from biological functions to therapeutic strategies
Experimental Hematology & Oncology (2023)
-
Regulatory T cells in skin regeneration and wound healing
Military Medical Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.