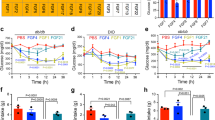
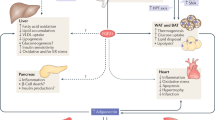
Abstract
Fibroblast growth factor 1 (FGF1) is an autocrine/paracrine regulator whose binding to heparan sulphate proteoglycans effectively precludes its circulation1,2. Although FGF1 is known as a mitogenic factor, FGF1 knockout mice develop insulin resistance when stressed by a high-fat diet, suggesting a potential role in nutrient homeostasis3,4. Here we show that parenteral delivery of a single dose of recombinant FGF1 (rFGF1) results in potent, insulin-dependent lowering of glucose levels in diabetic mice that is dose-dependent but does not lead to hypoglycaemia. Chronic pharmacological treatment with rFGF1 increases insulin-dependent glucose uptake in skeletal muscle and suppresses the hepatic production of glucose to achieve whole-body insulin sensitization. The sustained glucose lowering and insulin sensitization attributed to rFGF1 are not accompanied by the side effects of weight gain, liver steatosis and bone loss associated with current insulin-sensitizing therapies. We also show that the glucose-lowering activity of FGF1 can be dissociated from its mitogenic activity and is mediated predominantly via FGF receptor 1 signalling. Thus we have uncovered an unexpected, neomorphic insulin-sensitizing action for exogenous non-mitogenic human FGF1 with therapeutic potential for the treatment of insulin resistance and type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beenken, A. & Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nature Rev. Drug Discov. 8, 235–253 (2009)
Itoh, N. & Ornitz, D. M. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J. Biochem. 149, 121–130 (2011)
Jonker, J. W. et al. A PPARγ–FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature 485, 391–394 (2012)
Sun, K. & Scherer, P. E. The PPARγ–FGF1 axis: an unexpected mediator of adipose tissue homeostasis. Cell Res. 22, 1416–1418 (2012)
Lehrke, M. & Lazar, M. A. The many faces of PPARγ. Cell 123, 993–999 (2005)
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 (2005)
Dutchak, P. A. et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148, 556–567 (2012)
Zinn, K. R. et al. Imaging Tc-99m-labeled FGF-1 targeting in rats. Nucl. Med. Biol. 27, 407–414 (2000)
Lee, J. & Blaber, M. The interaction between thermodynamic stability and buried free cysteines in regulating the functional half-life of fibroblast growth factor-1. J. Mol. Biol. 393, 113–127 (2009)
Wu, A. L. et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci. Transl. Med. 3, 113ra126 (2011)
Li, A. J., Tsuboyama, H., Komi, A., Ikekita, M. & Imamura, T. Strong suppression of feeding by a peptide containing both the nuclear localization sequence of fibroblast growth factor-1 and a cell membrane-permeable sequence. Neurosci. Lett. 255, 41–44 (1998)
Suzuki, S. et al. Feeding suppression by fibroblast growth factor-1 is accompanied by selective induction of heat shock protein 27 in hypothalamic astrocytes. Eur. J. Neurosci. 13, 2299–2308 (2001)
Sasaki, K. et al. Effects of fibroblast growth factors and related peptides on food intake by rats. Physiol. Behav. 56, 211–218 (1994)
Wei, W. et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc. Natl Acad. Sci. USA 109, 3143–3148 (2012)
Holland, W. L. et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17, 790–797 (2013)
Lin, Z. et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17, 779–789 (2013)
Hevener, A. L. et al. Muscle-specific Pparg deletion causes insulin resistance. Nature Med. 9, 1491–1497 (2003)
Van Dijk, T. H. et al. Quantification of hepatic carbohydrate metabolism in conscious mice using serial blood and urine spots. Anal. Biochem. 322, 1–13 (2003)
Acknowledgements
We thank L. Chong, J. Alvarez, S. Kaufman, B. Collins, X. Zhao, S. Liu, A. Jurdzinski, A. Bleeker, K. Bijsterveld, D. Oh and G. Bandyopadhyay for technical assistance, and L. Ong and C. Brondos for administrative assistance. Computed tomography was performed at the Veterans Medical Research Foundation. R.M.E. is a Howard Hughes Medical Institute Investigator at the Salk Institute and March of Dimes Chair, and is supported by National Institutes of Health (NIH) grants (DK057978, DK090962, HL088093, HL105278 and ES010337), the Glenn Foundation for Medical Research, the Leona M. and Harry B. Helmsley Charitable Trust, Ipsen/Biomeasure, the California Institute for Regenerative Medicine and The Ellison Medical Foundation. C.L. and M.D. are funded by the National Health and Medical Research Council (grants 512354, 632886 and 1043199); J.W.J. by the European Research Council (grant IRG-277169), the Human Frontier Science Program (CDA00013/2011-C), the Netherlands Organisation for Scientific Research (VIDI grant 016.126.338), the Dutch Digestive Foundation (grant WO 11-67) and the Dutch Diabetes Foundation (grant 2012.00.1537); J.M.O. by NIH grants (DK-033651, DK-074868, T32-DK-007494, DK-063491 and P01-DK054441-14A1) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through cooperative agreement of U54-HD-012303-25 as part of the specialized Cooperative Centers Program in Reproduction and Infertility Research; M.M. by the National Institute of Dental and Craniofacial Research grant (DE13686); and M.A. by an F32 Ruth L. Kirschstein National Research Service Award (National Institute of Diabetes and Digestive and Kidney Diseases).
Author information
Authors and Affiliations
Contributions
J.M.S., J.W.J. M.D. and R.M.E. designed and supervised the research. J.M.S., J.W.J., M.A., R.G., D.L., O.O., Z.H., W.L., E.Y., T.H.D., R.H., W.F., Y.-Q.Y. and A.R.A. performed research. J.M.S., J.W.J., M.A., R.T.Y., C.L., A.R.A., J.M.O., M.M., M.D. and R.M.E. analysed data. J.M.S., J.W.J., M.A., R.G., A.R.A., M.D. and R.M.E. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Acute rFGF1 injection lowers blood glucose levels and reduces food intake.
a–c, Blood glucose levels in ob/ob mice after subcutaneous (sc, n = 3) or intraperitoneal (ip, n = 3) injection of rFGF1 (a), intravenous (iv) injection of rFGF1 (n = 6) or control vehicle (n = 5) (b), or subcutaneous injection of rFGF1 premixed with heparin (1.5 mg kg−1, n = 3) (c). d, Serum insulin levels 24 h after rFGF1 treatment under ad libitum fed or fasting conditions in chow-fed C57BL/6J mice (n = 10). e, Blood glucose levels in fasted, chow-fed mice after treatment with rFGF1 (n = 8). f, Food intake during 24 h after injection of rFGF1 in chow-fed C57BL/6J mice (n = 8). g–i, Food intake during indicated times after injection of rFGF1 in ob/ob (n = 6) (g), db/db (n = 3) (h) and DIO (n = 6) (i) mice. Control PBS (open bar) or murine rFGF1 (0.5 mg kg−1; filled bar) were injected subcutaneously to ad libitum fed mice unless otherwise noted. Values are means and s.e.m. Statistics by two-tailed t-test: *P < 0.05; **P < 0.01.
Extended Data Figure 2 Chronic administration of rFGF1 lowers blood glucose independently of food intake and metabolic hormones.
a–c, MRI analyses of fat (a) and lean mass content (b) at indicated days, and food intake (c) during chronic administration of control vehicle (n = 6) or rFGF1 (n = 8) in ob/ob mice. d, Random-fed blood glucose of a pair-fed cohort of ob/ob mice (red line, filled triangles, n = 12) plotted alongside blood glucose trends during chronic administration of control vehicle (black line, open squares, n = 6) or rFGF1 in ob/ob mice (black line, filled squares; n = 8). Pair-fed cohort food intake was restricted to equal the food intake of rFGF1-injected ob/ob mice throughout the 5-week trial. e–g, Serum cholesterol (e), free fatty acids (f) and metabolic hormone levels (g) after 5-week administration of control PBS or rFGF1 in ob/ob mice (n = 4). h–j, Body weight (h), tissue mass analyses (i) and food intake (j) of DIO mice after 4 weeks of rFGF1 treatment (n = 6). All injections were performed subcutaneously with control vehicle (PBS, open bars or symbols) or murine rFGF1 (0.5 mg kg−1; filled bars or symbols) to ad libitum fed mice every 48 h throughout chronic administration trials. Values are means and s.e.m. Statistics by two-tailed t-test: *P < 0.05; **P < 0.01.
Extended Data Figure 3 rFGF1 does not stimulate insulin secretion and chronic administration reduces systemic inflammation.
a, Glucose-stimulated insulin secretion (basal; 3 mM glucose; stimulated, 20 mM glucose) of ob/ob islets after 1 h pretreatment with control PBS (open bars) or rFGF1 (10 ng ml−1, filled bars; n = 6). b, c, Time course of serum insulin (b) and blood glucose (c) levels in ob/ob mice after a single rFGF1 injection (0.2 mg kg−1 intravenously; n = 8). d, Serum cytokines in ob/ob mice after 5 weeks of subcutaneous administration of control vehicle (PBS, open bars; n = 4) or rFGF1 (0.5 mg kg−1 every other day, filled bars; n = 6) Values are means and s.e.m. Statistics by two-tailed t-test: *P < 0.05; **P < 0.01.
Extended Data Figure 4 rFGF1 is an insulin sensitizer and does not affect bone morphology.
a–d, Steady-state glucose infusion rate (a), hepatic inflammation-related gene expression (b), glucose tolerance tests (c) and body weights (d) of DIO mice after 3 weeks of administration of control PBS (n = 11) or rFGF1 (0.5 mg kg−1 every other day; n = 9). e, f, Basal hepatic glucose production (e) and basal and clamped serum insulin concentrations (f) measured during hyperinsulinaemic–euglycaemic clamp studies of DIO mice after 3 weeks of administration of control PBS (n = 11) or rFGF1 (n = 9). g, h, Insulin induced phosphorylation of AKT in liver (g) and muscle tissues (h) of DIO mice after 3 weeks of administration of control PBS (n = 11) or rFGF1 (n = 12). i–n, Food intake (i), carbon dioxide production (j), heat production (k), total activity (l), respiratory exchange ratio (m) and oxygen consumption (n) of chronically rFGF1-treated DIO mice (3 weeks of treatment with control PBS (blue symbols, n = 4) or rFGF1 (red symbols, n = 4) measured in metabolic cages. o, Representative haematoxylin and eosin staining of inguinal white adipose tissue from DIO mice after 4 weeks of administration of control PBS (n = 6) or rFGF1 (n = 6). p, Serum creatine kinase levels in chronically rFGF1-treated ob/ob mice (n = 4). q, r, Bone mineral density (q) and trabecular bone thickness (Tb Th), trabecular bone space (Tb Sp) and cortical bone thickness (C Th) (r) in 4-week treated DIO mice analysed by micro-computed tomography (n = 6). s, Total and high-molecular-weight (HMW) serum adiponectin levels in ob/ob mice after 4 weeks of rFGF1 injections every 48 h (n = 4). Control vehicle (PBS, open bar), rFGF1 (0.5 mg kg−1 subcutaneously; filled bars). Scale bar, 100 μm. Values are means and s.e.m. Statistics by two-tailed t-test: *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001.
Extended Data Figure 5 Binding affinities of rFGF1 and rFGF1ΔNT to FGFRs.
Removal of the N terminus from FGF1 reduces the ligand’s binding affinity for FGFRs. a, Overlays of SPR sensorgrams of FGF1 binding to the ligand-binding domain of FGFRs and fitted saturation binding curves. Equilibrium dissociation constants (Kd values) were derived from the saturation binding curves. b, Overlays of SPR sensorgrams of FGF1ΔNT binding to the ligand-binding domain of FGFRs. Where possible, Kd values were calculated from fitted saturation binding curves.
Extended Data Figure 6 rFGF1 and rFGF1ΔNT signal through FGFR1 in a dose-dependent manner.
a, Western blot showing intracellular signalling in serum-starved HEK293 cells after a 15-min treatment with the indicated concentrations of PBS, rFGF1ΔNT or rFGF1. b, Dose response of glucose-lowering effects of subcutaneously delivered rFGF1ΔNT (striped bars) in comparison with rFGF1 (filled bars) in 12-week-old ob/ob mice (n = 8). c, Food intake in DIO mice during a 24 h period after injection of control PBS (open bar), rFGF1 (0.5 mg kg−1 subcutaneously; filled bars) or rFGF1ΔNT (0.5 mg kg−1 subcutaneously; striped bar, n = 10). d, Blood glucose levels in high-fat-diet-fed (8 months) Fgfr1fl/fl (WT, n = 5) and aP2-Cre;Fgfr1fl/fl (R1 knockout (KO), n = 4) mice at 0 h (open bars) and 24 h (filled bars) after treatment with rFGF1ΔNT (0.5 mg kg−1 subcutaneously). e, Western blot showing intracellular signalling in serum-starved HEK293 cells after a 15-min treatment with PBS or 10 ng ml−1 rFGF1, two independent preparations of rFGF1ΔNT, and rFGF1ΔNT2. f, Blood glucose levels in ob/ob mice at 0 h (open bars) and 24 h (filled bars) after treatment with rFGF1ΔNT and rFGF1ΔNT2 (0.5 mg kg−1 subcutaneously; n = 2). Gel images are representative of at least three biological replicates. Values are means and s.e.m. Statistics by two-tailed t-test: *P < 0.05; ***P < 0.005.
Rights and permissions
About this article
Cite this article
Suh, J., Jonker, J., Ahmadian, M. et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513, 436–439 (2014). https://doi.org/10.1038/nature13540
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13540
This article is cited by
-
Sensory nerve niche regulates mesenchymal stem cell homeostasis via FGF/mTOR/autophagy axis
Nature Communications (2023)
-
Intracellular FGF1 protects cells from apoptosis through direct interaction with p53
Cellular and Molecular Life Sciences (2023)
-
The ventromedial hypothalamic nucleus: watchdog of whole-body glucose homeostasis
Cell & Bioscience (2022)
-
FGF1 alleviates LPS-induced acute lung injury via suppression of inflammation and oxidative stress
Molecular Medicine (2022)
-
Metabolic Messengers: fibroblast growth factor 1
Nature Metabolism (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.