Abstract
The proton gradient is a principal energy source for respiration-dependent active transport, but the structural mechanisms of proton-coupled transport processes are poorly understood. YiiP is a proton-coupled zinc transporter found in the cytoplasmic membrane of Escherichia coli. Its transport site receives protons from water molecules that gain access to its hydrophobic environment and transduces the energy of an inward proton gradient to drive Zn(ii) efflux1,2. This membrane protein is a well-characterized member3,4,5,6,7 of the family of cation diffusion facilitators that occurs at all phylogenetic levels8,9,10. Here we show, using X-ray-mediated hydroxyl radical labelling of YiiP and mass spectrometry, that Zn(ii) binding triggers a highly localized, all-or-nothing change of water accessibility to the transport site and an adjacent hydrophobic gate. Millisecond time-resolved dynamics reveal a concerted and reciprocal pattern of accessibility changes along a transmembrane helix, suggesting a rigid-body helical re-orientation linked to Zn(ii) binding that triggers the closing of the hydrophobic gate. The gated water access to the transport site enables a stationary proton gradient to facilitate the conversion of zinc-binding energy to the kinetic power stroke of a vectorial zinc transport. The kinetic details provide energetic insights into a proton-coupled active-transport reaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lu, M., Chai, J. & Fu, D. Structural basis for autoregulation of the zinc transporter YiiP. Nature Struct. Mol. Biol. 16, 1063–1067 (2009)
Grass, G. et al. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 183, 9–18 (2005)
Chao, Y. & Fu, D. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. J. Biol. Chem. 279, 17173–17180 (2004)
Wei, Y. & Fu, D. Selective metal binding to a membrane-embedded aspartate in the Escherichia coli metal transporter YiiP (FieF). J. Biol. Chem. 280, 33716–33724 (2005)
Wei, Y. & Fu, D. Binding and transport of metal ions at the dimer interface of the Escherichia coli metal transporter YiiP. J. Biol. Chem. 281, 23492–23502 (2006)
Wei, Y., Li, H. & Fu, D. Oligomeric state of the Escherichia coli metal transporter YiiP. J. Biol. Chem. 279, 39251–39259 (2004)
Chao, Y. & Fu, D. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J. Biol. Chem. 279, 12043–12050 (2004)
Kambe, T., Yamaguchi-Iwai, Y., Sasaki, R. & Nagao, M. Overview of mammalian zinc transporters. Cell. Mol. Life Sci. 61, 49–68 (2004)
Montanini, B., Blaudez, D., Jeandroz, S., Sanders, D. & Chalot, M. Phylogenetic and functional analysis of the cation diffusion facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics 8, 107 (2007)
Nies, D. H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27, 313–339 (2003)
Palmiter, R. D., Cole, T. B., Quaife, C. J. & Findley, S. D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl Acad. Sci. USA 93, 14934–14939 (1996)
Lemaire, K. et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl Acad. Sci. USA 106, 14872–14877 (2009)
Lu, M. & Fu, D. Structure of the zinc transporter YiiP. Science 317, 1746–1748 (2007)
Xu, G. & Chance, M. R. Hydroxyl radical-mediated modification of proteins as probes for structural proteomics. Chem. Rev. 107, 3514–3543 (2007)
Takamoto, K. & Chance, M. R. Radiolytic protein footprinting with mass spectrometry to probe the structure of macromolecular complexes. Annu. Rev. Biophys. Biomol. Struct. 35, 251–276 (2006)
Gupta, S. et al. Conformational changes during the gating of a potassium channel revealed by structural mass spectrometry. Structure 18, 839–846 (2010)
Angel, T. E., Gupta, S., Jastrzebska, B., Palczewski, K. & Chance, M. R. Structural waters define a functional channel mediating activation of the GPCR, rhodopsin. Proc. Natl Acad. Sci. USA 106, 14367–14372 (2009)
Gupta, S., D’Mello, R. & Chance, M. R. Structure and dynamics of protein waters revealed by radiolysis and mass spectrometry. Proc. Natl Acad. Sci. USA 109, 14882–14887 (2012)
Podar, D. et al. Metal selectivity determinants in a family of transition metal transporters. J. Biol. Chem. 287, 3185–3196 (2012)
Lin, H. et al. Gain-of-function mutations identify amino acids within transmembrane domains of the yeast vacuolar transporter Zrc1 that determine metal specificity. Biochem. J. 422, 273–283 (2009)
Coudray, N. et al. Inward-facing conformation of the zinc transporter YiiP revealed by cryoelectron microscopy. Proc. Natl Acad. Sci. USA 110, 2140–2145 (2013)
Hinds, B. J. et al. Aligned multiwalled carbon nanotube membranes. Science 303, 62–65 (2004)
Hoch, E. et al. Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc. Natl Acad. Sci. USA 109, 7202–7207 (2012)
Ramos, S., Schuldiner, S. & Kaback, H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc. Natl Acad. Sci. USA 73, 1892–1896 (1976)
Gupta, S., Sullivan, M., Toomey, J., Kiselar, J. & Chance, M. R. The Beamline X28C of the Center for Synchrotron Biosciences: a national resource for biomolecular structure and dynamics experiments using synchrotron footprinting. J. Synchrotron Radiat. 14, 233–243 (2007)
Kaur, P., Kiselar, J. G. & Chance, M. R. Integrated algorithms for high-throughput examination of covalently labeled biomolecules by structural mass spectrometry. Anal. Chem. 81, 8141–8149 (2009)
Ralston, C. Y. et al. Time-resolved synchrotron X-ray footprinting and its application to RNA folding. Methods Enzymol. 317, 353–368 (2000)
Acknowledgements
We dedicate this work to the memory of Peter C. Maloney, who read and commented on a version of the manuscript. This work was supported in part by the National Institutes of Health under grant R01 GM065137 (to D.F.), the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the US Department of Energy (DOE) under contract DE-AC02-98CH10886 (to D.F.); D.F. is primarily supported by the Physical Biosciences Program, Office of Basic Energy Sciences of the DOE and the National Institute for Biomedical Imaging and Bioengineering under grants P30-EB-09998 and R01-EB-09688 (to M.R.C.). The National Synchrotron Light Source at Brookhaven National Laboratory is supported by the DOE under contract DE-AC02-98CH10886.
Author information
Authors and Affiliations
Contributions
M.R.C. and D.F. conceived the work, S.G. and D.F. designed the experiments, S.G., J.C., R.D. and D.F. performed the experiments, S.G., M.R.C. and D.F analysed the data, and D.F. interpreted the data and wrote the paper with S.G. and M.R.C.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Steady state and time-resolved synchrotron X-ray radiolysis.
a, Experimental scheme for obtaining steady state dose plots. b, Experimental schemes for obtaining the time course of water accessibility change.
Extended Data Figure 2 Proteomics sequence coverage of YiiP.
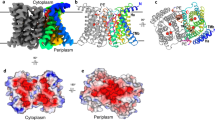
a, Mass spectrometry sequence coverage. The detectable peptides, undetectable residues and coordination residues of the transport site are shown in orange, green and red, respectively. Transmembrane helixes (TMs) are underlined as indicated. b, Mapping detectable proteolytic peptides to the YiiP crystal structure. Detectable and undetectable peptides in one protomer of a YiiP homodimer are coloured in orange and green, respectively. The side chains of detectable residues located between two cavities are shown in surface representation and coloured in red. Bound zinc ions are shown as magenta spheres.
Extended Data Figure 3 Dose–responses for modified sites in apo-YiiP (red) and zinc-YiiP (black).
Solid lines represent least-squares fits of the means of the dose-dependent data as described in Methods. The reactivity rate for each site as indicated is summarized in Extended Data Table 1. Note, the linear dose–response on a logarithm scale indicates negligible radiation damage by 10 ms irradiation to all sites except M151, M197 and P286C287, which reached saturation at 5 ms. In these cases, the 10 ms data points were not used in linear regression. Dose–response plots for M151 and L152 are shown in Fig. 1d. Zinc binding to a solvent-exposed zinc site located on the cytoplasmic membrane surface (Z2, Fig. 2b) yielded 38–67% reductions in oxidative modification of neighbouring L64, P66, D68, D69, H71 and F73 within the peptide SLQPADDNHSF (Extended Data Table 1). A marginal 20% reduction in water accessibility change by zinc binding was also localized to W172 and W175 within an extracellular loop connecting TM5 and TM6 (Fig. 2b). This loop is disordered in the crystal structure. Structural flexibility probably allows for distinct loop conformations in response to zinc binding. A total of six modified sites were identified in CTD (Extended Data Table 1). Two sites (M251 and P286/C287) with reduced water accessibility for zinc-YiiP are located near the binuclear zinc-binding site at the CDT–CDT interface. Zinc binding may partly protect these sites from labelling, but the protection was incomplete because of the solvent exposure. Two sites on the CTD protein surface (W225 and P257/L258) showed no detectable change as expected for fully exposed residues. Still two more sites exhibited an increase in water accessibility for zinc-YiiP. The first site (D207–L210) was detected with low signal-to-noise ratio and mapped to a TMD-CDT linker, which is involved in a hinge-like conformational change in response to zinc binding1. The second site involved M262 on the CDT surface. There was no obvious explanation for the increase of water accessibility to this methionine residue.
Extended Data Figure 4 Sequence conservation of the inter-cavity seal.
Residues involved in TM5→TM3–TM6 packing are marked by black asterisks in a CDF sequence alignment. Conserved and homologous residues are coloured in magenta and light brown. Magenta, cyan and black dots indicate residues involved in zinc coordination, dimerization contacts and the interlocked (Lys 77–Asp 207)2 salt-bridges, respectively. Dashed lines in two human ZnT sequences represent omitted residues in a loop (IL2) between TM4 and TM5. The red arrow indicates the position of the R325W mutation in human ZnT8.
Extended Data Figure 5 Expression and size-exclusion analysis of purified L152 mutants.
L152 was substituted by an A, D, F, G, I, M or R residue to evaluate the effect of L152 mutations on structural stability. a, Western blot analysis of the expression of YiiP and L152 mutants as indicated. Only a L152R point mutation caused a modest reduction of protein expression, whereas other L152 substitutions and wild-type YiiP showed a similar level of expression based on western blot detection of His-tagged proteins in membrane vesicles using a monoclonal antibody against the poly-histidine tag. b, Eluted protein peaks for YiiP and L152 mutants as indicated after the removal of protein aggregates by ultracentrifugation. YiiP and L152 mutants were solubilized by DDM and purified by Ni-NTA affinity chromatography and size-exclusion HPLC. The purified wild-type YiiP remained stable in DDM micelles for weeks. Sizing HPLC analysis showed a monodisperse YiiP peak followed by a minor detergent peak eluted at expected retention times6. In sharp contrast, purified L152 mutants rapidly denatured, forming lager protein aggregates. After removing the aggregates by ultracentrifugation, none of the purified L152A, D, F, G and R became detectable by sizing HPLC. Two conserved L152 substitutions, L152I and L152M, showed a significantly reduced peak volume within 48 h of DDM solubilization. A prolonged DDM solubilization led to complete denaturation of L152I and L152M while the wild-type YiiP remained stable under the same experimental conditions. Thus, L152 is critically important to the protein stability in detergent micelles. The lack of protein stability in detergent solution precluded functional reconstitution and characterization of purified L152 mutants.
Rights and permissions
About this article
Cite this article
Gupta, S., Chai, J., Cheng, J. et al. Visualizing the kinetic power stroke that drives proton-coupled zinc(ii) transport. Nature 512, 101–104 (2014). https://doi.org/10.1038/nature13382
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13382
This article is cited by
-
Somatic SLC30A1 mutations altering zinc transporter ZnT1 cause aldosterone-producing adenomas and primary aldosteronism
Nature Genetics (2023)
-
An automated liquid jet for fluorescence dosimetry and microsecond radiolytic labeling of proteins
Communications Biology (2022)
-
Nanoparticles and photochemistry for native-like transmembrane protein footprinting
Nature Communications (2021)
-
Exposure of Solvent-Inaccessible Regions in the Amyloidogenic Protein Human SOD1 Determined by Hydroxyl Radical Footprinting
Journal of the American Society for Mass Spectrometry (2019)
-
Evaluation of the roles of the cytosolic N-terminus and His-rich loop of ZNT proteins using ZNT2 and ZNT3 chimeric mutants
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.